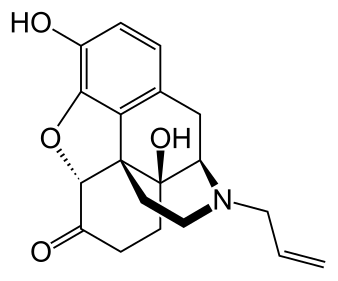
Friday, December 19, 2025
FDA Approves KETARx (ketamine) for Surgical Pain Management
Friday, September 13, 2024
FDA Approves Rezenopy (naloxone hydrochloride) Nasal Spray for the Emergency Treatment of Opioid Overdose
Naloxone hydrochloride is an opioid antagonist that works to reverse the effects of opioids during an overdose, including respiratory depression, sedation and hypotension.
Rezenopy is a high-dose naloxone hydrochloride nasal spray formulation containing 10 mg of naloxone per spray available on prescription. There are a number of naloxone hydrochloride nasal spray products available that contain a lower dose of naloxone, including Kloxxado (8 mg/spray) and Rextovy (4 mg/spray) which are available on prescription, and Narcan (4 mg/spray) and ReVive (3 mg/spray) which are available over-the-counter.
Thursday, August 8, 2024
Defender Pharmaceuticals Receives Complete Response Letter from the U.S. Food and Drug Administration for its Intranasal Scopolamine (DPI-386) New Drug Application for the Prevention of Nausea and Vomiting Induced by Motion in Adults
Certain motions cause discomfort in individuals while engaged in various leisure or travel-related activities. Most forms of travel, whether on land, in the air, or on the water, can trigger symptoms such as nausea and vomiting (example: flying, boating/fishing, car, bus, and train). Symptoms induced by motion can also have a detrimental impact on the ability of various military personnel and astronauts to perform assigned duties, potentially impacting readiness and negatively impacting resources. Motion-related discomfort is a common and transient response to unfamiliar or unnatural motion or contradictory spatial sensory information, resulting in decrements to performance of tasks, pallor, cold sweating, nausea and vomiting. Prolonged exposure to certain motions may induce sopite-related symptoms such as loss of drive and concentration, drowsiness, sleepiness, apathy, depression, and a feeling of impending doom.
Friday, September 30, 2022
FDA Approves Auvelity (dextromethorphan and bupropion) for the Treatment of Major Depressive Disorder in Adults
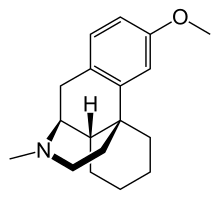

In continuation of my updates on dextromethorphan and Bupropion,
Axsome Therapeutics, Inc. , a biopharmaceutical company developing and delivering novel therapies for the management of central nervous system (CNS) disorders, announced the U.S. Food and Drug Administration (FDA) approval of Auvelity (dextromethorphan HBr -bupropion HCl) extended-release tablets for the treatment of major depressive disorder (MDD) in adults.1 Auvelity is the first and only rapid-acting oral medicine approved for the treatment of MDD with labeling of statistically significant antidepressant efficacy compared to placebo starting at one week. The rapid antidepressant effects of Auvelity were sustained at all subsequent timepoints. Auvelity is the first and only oral N-methyl D-aspartate (NMDA) receptor antagonist approved for the treatment of MDD. Axsome anticipates Auvelity to be commercially available in the U.S. in the fourth quarter of 2022.
Maurizio Fava, MD, Psychiatrist-In-Chief, Department of Psychiatry, Massachusetts General Hospital, Executive Director, Clinical Trials Network & Institute, Associate Dean for Clinical & Translational Research, and Slater Family Professor of Psychiatry, Harvard Medical School said, “The approval of Auvelity represents a milestone in depression treatment based on its novel oral NMDA antagonist mechanism, its rapid antidepressant efficacy demonstrated in controlled trials, and a relatively favorable safety profile. Auvelity, which was granted Breakthrough Therapy designation by the FDA, represents the first new oral non-monoamine-based mechanism of action approved to treat major depressive disorder in over sixty years. Nearly two thirds of patients treated with currently available antidepressants do not adequately respond, and those that do may not achieve clinically meaningful responses for up to six to eight weeks. Given the debilitating nature of depression, the efficacy of Auvelity observed at one week and sustained thereafter may have a significant impact on the current treatment paradigm for this condition.”
Michael Pollock, Chief Executive Officer of the Depression and Bipolar Support Alliance (DBSA), a leading national patient advocacy organization focusing on depression and bipolar disorder said, “The mental health crisis in the United States is one of the most pressing health issues facing our country today. Over 20 million American adults experienced major depressive disorder each year prior to the COVID-19 pandemic. These numbers increased dramatically during the pandemic with approximately thirty percent of adults in the U.S. or more than 80 million Americans experiencing elevated symptoms of depression. The need for new treatment options, particularly those with new mechanisms of action, could not be clearer and more urgent for those living with, or impacted by, major depressive disorder.”
Dan V. Iosifescu, MD, Professor of Psychiatry at the New York University School of Medicine, and Director of the Clinical Research Division at the Nathan Kline Institute for Psychiatric Research said, “Major depressive disorder is disabling and potentially life-threatening, causes profound distress for patients and their families, and leads to substantial healthcare resource utilization. Auvelity’s oral NMDA receptor antagonist and sigma-1 receptor agonist activity, which targets glutamatergic neurotransmission, provides clinicians a long sought after new mechanistic approach which may benefit the millions of patients living with this serious condition. In clinical trials, Auvelity has demonstrated rapid and statistically significant improvement in depressive symptoms as early as Week 1, and increased rates of remission at Week 2 compared with placebo. This early benefit with Auvelity was maintained and increased with continued treatment, and was accompanied by a favorable safety and tolerability profile.”
Auvelity was studied in a comprehensive clinical program which included more than 1,100 patients with depression. The efficacy of Auvelity in the treatment of MDD was demonstrated in the GEMINI placebo-controlled study, and confirmatory evidence which included the ASCEND study comparing Auvelity to bupropion sustained-release tablets. In the GEMENI study, Auvelity was statistically significantly superior to placebo in improvement of depressive symptoms as measured by the change in the Montgomery-Åsberg Depression Rating Scale (MADRS) total score at Week 6, the study’s primary endpoint. To evaluate speed of onset of action, the change in MADRS total score from baseline to Week 1 and from baseline to Week 2 were pre-specified secondary efficacy endpoints. The difference between Auvelity and placebo in change from baseline in MADRS total score was statistically significant at Week 1 and at Week 2.1 In the ASCEND study, Auvelity was statistically significantly superior to bupropion sustained-release tablets 105 mg twice daily on the primary outcome measure.5 The primary outcome measure of the ASCEND study was calculated by assessing the change from baseline in MADRS total scores from Week 1 to Week 6 and then taking the average of those scores.1 In the placebo-controlled clinical study, the most common (incidence ≥5% for Auvelity and more than twice as frequently as placebo) adverse reactions were dizziness, headache, diarrhea, somnolence, dry mouth, sexual dysfunction, and hyperhidrosis.1
The FDA granted Breakthrough Therapy designation for Auvelity for the treatment of MDD in March 2019. This designation is granted to candidate drugs that show potential for benefit above that of available therapies based on preliminary clinical data, and it provides the sponsor with added focus from and greater interactions with FDA staff during the development of the candidate drug.6 The Auvelity New Drug Application (NDA) was evaluated by the FDA under Priority Review, which is granted by the FDA to applications for medicines that, if approved, would provide significant improvements in the effectiveness or safety of the treatment, diagnosis, or prevention of serious conditions when compared to standard applications.
https://en.wikipedia.org/wiki/Bupropion
https://en.wikipedia.org/wiki/Dextromethorphan
Monday, June 28, 2021
Drug commonly used as antidepressant helps fight cancer in mice
A new study by UCLA researchers suggests that those drugs, commonly known as MAOIs, might have another health benefit: helping the immune system attack cancer. Their findings are reported in two papers, which are published in the journals Science Immunology and Nature Communications.
"MAOIs had not been linked to the immune system's response to cancer before," said Lili Yang, senior author of the study and a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA. "What's especially exciting is that this is a very well-studied and safe class of drug, so repurposing it for cancer isn't as challenging as developing a completely new drug would be."
Recent advances in understanding how the human immune system naturally seeks out and destroys cancer cells, as well as how tumors try to evade that response, has led to new cancer immunotherapies—drugs that boost the immune system's activity to try to fight cancer.
In an effort to develop new cancer immunotherapies, Yang and her colleagues compared immune cells from melanoma tumors in mice to immune cells from cancer-free animals. Immune cells that had infiltrated tumors had much higher activity of a gene called monoamine oxidase A, or MAOA. MAOA's corresponding protein, called MAO-A, controls levels of serotonin and is targeted by MAOI drugs.
"For a long time, people have theorized about the cross-talk between the nervous system and the immune system and the similarities between the two," said Yang, who is also a UCLA associate professor of microbiology, immunology and molecular genetics and a member of the UCLA Jonsson Comprehensive Cancer Center. "So it was exciting to find that MAOA was so active in these tumor-infiltrating immune cells."
Next, the researchers studied mice that didn't produce MAO-A protein in immune cells. The scientists found that those mice were better at controlling the growth of melanoma and colon tumors. They also found that normal mice became more capable of fighting those cancers when treated with MAOIs.
Digging in to the effects of MAO-A on the immune system, the researchers discovered that T cells—the immune cells that target cancer cells for destruction—produce MAO-A when they recognize tumors, which diminishes their ability to fight cancer.
That discovery places MAO-A among a growing list of molecules known as immune checkpoints, which are molecules produced as part of a normal immune response to prevent T cells from overreacting or attacking healthy tissue in the body. Cancer has been known to exploit the activity of other previously identified immune checkpoints to evade attack by the immune system.
In the Science Immunology paper, the scientists report that MAOIs help block the function of MAO-A, which helps T cells overcome the immune checkpoint and more effectively fight the cancer.
But the drugs also have a second role in the immune system, Yang found. Rogue immune cells known as tumor-associated macrophages often help tumors evade the immune system by preventing anti-tumor cells including T cells from mounting an effective attack. High levels of those immunosuppressive tumor-associated macrophages in a tumor have been associated with poorer prognoses for people with some types of cancer.
But the researchers discovered that MAOIs block immunosuppressive tumor-associated macrophages, effectively breaking down one line of defense that tumors have against the human immune system. That finding is reported in the Nature Communications paper.
"It turns out that MAOIs seem to both directly help T cells do their job, and stop tumor-associated macrophages from putting the brakes on T cells," Yang said.
Combining MAOIs with existing immunotherapies
Yang said she suspects that MAOIs may work well in concert with a type of cancer immunotherapies called immune checkpoint blockade therapies, most of which work by targeting immune checkpoint molecules on the surface of immune cells. That's because MAOIs work on MAO-A proteins, which are inside cells and function differently from other known immune checkpoint molecules.
Studies in mice showed that any of three existing MAOIs—phenelzine, clorgyline or mocolobemide—either on their own or in combination with a form of immune checkpoint blockade therapy known as PD-1 blockers, could stop or slow the growth of colon cancer and melanoma.
Although they haven't tested the drugs in humans, the researchers analyzed clinical data from people with melanoma, colon, lung, cervical and pancreatic cancer; they found that people with higher levels of MAOA gene expression in their tumors had, on average, shorter survival times. That suggests that targeting MAOA with MAOIs could potentially help treat a broad range of cancers.
Yang and her collaborators are already planning additional studies to test the effectiveness of MAOIs in boosting human immune cells' response to various cancers.
Yang said MAOIs could potentially act on both the brain and immune cells in patients with cancer, who are up to four times as likely as the general population to experience depression.
"We suspect that repurposing MAOIs for cancer immunotherapy may provide patients with dual antidepressant and antitumor benefits," she said.
The experimental combination therapy in the study was used in preclinical tests only and has not been studied in humans or approved by the Food and Drug Administration as safe and effective for use in humans. The newly identified therapeutic strategy is covered by a patent application filed by the UCLA Technology Development Group on behalf of the Regents of the University of California, with Yang, Xi Wang and Yu-Chen Wang as co-inventors.
Thursday, April 1, 2021
Common drug could mitigate risk of 'a broken heart' during bereavement
About the study
The main finding was that the active medication, used in a low dose once a day, successfully reduced spikes in blood pressure and heart rate, as well as demonstrating some positive change in blood clotting tendency."Professor Geoffrey Tofler, lead investigator
Implications and next steps
This is an important study because it shows ways to improve the physical and mental health of at-risk bereaved people. It is a preventive intervention that is potentially practice-changing, using inexpensive, commonly available medicines."
Monday, December 14, 2020
Fluvoxamine may prevent serious illness in COVID-19 patients, study suggests: Antidepressant drug repurposed for patients with coronavirus infection
The study, a collaboration between the university's Department of Psychiatry and Division of Infectious Diseases, involved 152 patients infected with SARS-CoV-2, the virus that causes COVID-19. Researchers compared the outcomes of those treated with fluvoxamine to the outcomes of those given an inactive placebo. After 15 days, none of the 80 patients who had received the drug experienced serious clinical deterioration. Meanwhile, six of the 72 patients given placebo (8.3%) became seriously ill, with four requiring hospitalization.
The study is published online Nov. 12 in the Journal of the American Medical Association.
"The patients who took fluvoxamine did not develop serious breathing difficulties or require hospitalization for problems with lung function," said the paper's first author, Eric J. Lenze, MD, the Wallace and Lucille Renard Professor of Psychiatry. "Most investigational treatments for COVID-19 have been aimed at the very sickest patients, but it's also important to find therapies that prevent patients from getting sick enough to require supplemental oxygen or to have to go to the hospital. Our study suggests fluvoxamine may help fill that niche."
Fluvoxamine is used commonly to treat obsessive-compulsive disorder (OCD), social anxiety disorder and depression. It is in a class of drugs known as selective serotonin-reuptake inhibitors (SSRIs), but unlike other SSRIs, fluvoxamine interacts strongly with a protein called the sigma-1 receptor. That receptor also helps regulate the body's inflammatory response.
"There are several ways this drug might work to help COVID-19 patients, but we think it most likely may be interacting with the sigma-1 receptor to reduce the production of inflammatory molecules," said senior author Angela M Reiersen, MD, an associate professor of psychiatry. "Past research has demonstrated that fluvoxamine can reduce inflammation in animal models of sepsis, and it may be doing something similar in our patients."
Reiersen said the drug's effects on inflammation could prevent the immune system from mounting an overwhelming response, which is thought to occur in some COVID-19 patients who seem to improve after a few days of illness and then worsen. Many of those patients end up hospitalized, and some die.
In an innovative twist to research during the pandemic, the study was conducted remotely. When a symptomatic patient tested positive and enrolled in the study, research staff delivered the medication or inactive placebo to them, along with thermometers, automatic blood pressure monitors and fingertip oxygen sensors.
"Our goal is to help patients who are initially well enough to be at home and to prevent them from getting sick enough to be hospitalized," said Caline Mattar, MD, an assistant professor of medicine in the Division of Infectious Diseases. "What we've seen so far suggests that fluvoxamine may be an important tool in achieving that goal."
For two weeks, subjects took either the antidepressant drug or placebo sugar pills while having daily interactions with members of the research team -- via phone or computer. That allowed patients to report on their symptoms, oxygen levels and other vital signs. If patients suffered shortness of breath or were hospitalized for pneumonia, or their oxygen saturation levels fell below 92%, their conditions were considered to have deteriorated.
"The good news is that not a single person taking the active medication experienced deterioration," Reiersen said. "We believe this drug may be the reason, but we need to study more patients to make sure."
The researchers will begin a larger study in the next few weeks. Lenze, the director of the Healthy Mind Lab at the School of Medicine, is an expert in using mobile and internet technology to conduct clinical trials. He said that although this initial study involved patients in the St. Louis region, the next phase of the research will involve patients from throughout the country.
"We bring the study to the patients, giving them tools to monitor their health at home," Lenze said. "Our hope is that we can keep these patients healthy enough to avoid hospitalization."
This work was supported by the Taylor Family Institute for Innovative Psychiatric Research, the Bantly Foundation, the Center for Brain Research in Mood Disorders at Washington University and the COVID-19 Early Treatment Fund. Additional support from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). Grant number UL1 TR002345.
Tuesday, November 17, 2020
Kratom Seems Safe for Pain, Anxiety, Opioid Withdrawal

https://www.sciencedirect.com/science/article/abs/pii/S0376871620300144
https://en.wikipedia.org/wiki/Mitragyna_speciosa
Friday, November 13, 2020
FDA Approves Isturisa (osilodrostat) for the Treatment of Cushing’s Disease
“The FDA supports the development of safe and effective treatments for rare diseases, and this new therapy can help people with Cushing’s disease, a rare condition where excessive cortisol production puts them at risk for other medical issues,” said Mary Thanh Hai, M.D., acting director of the Office of Drug Evaluation II in the FDA’s Center for Drug Evaluation and Research. “By helping patients achieve normal cortisol levels, this medication is an important treatment option for adults with Cushing’s disease.”
------------------------------------------------------------------------------------------------------------------
FDA Approves Isturisa (osilodrostat) for the Treatment of Cushing’s Disease
Thursday, July 9, 2020
A single dose of magic mushroom compound reduces anxiety and depression among cancer patients

Psilocybin effects
Promising results
Wednesday, April 29, 2020
FDA Acceptance of ALKS 3831 New Drug Application for Treatment of Schizophrenia and Bipolar Disorder
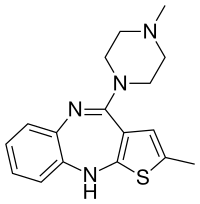
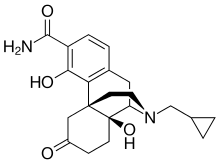
"The acceptance of the NDA for ALKS 3831 marks an important milestone toward our goal of offering a new treatment option to people living with schizophrenia or bipolar I disorder. The ALKS 3831 development program builds on Alkermes' commitment to developing new therapeutic options that seek to address unmet needs of patients in large therapeutic areas," said Craig Hopkinson, M.D., Chief Medical Officer at Alkermes. "We believe ALKS 3831 has the potential to be a meaningful new offering for patients with these serious and complex mental health disorders, and we look forward to engaging with the FDA throughout the NDA review process."
https://en.wikipedia.org/wiki/Olanzapine
https://en.wikipedia.org/wiki/Samidorphan
Friday, February 14, 2020
FDA Approves Caplyta (lumateperone) for the Treatment of Schizophrenia in Adults
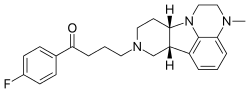
https://en.wikipedia.org/wiki/Lumateperone
Friday, January 31, 2020
Sunovion Announces Acceptance by the U.S. FDA of the New Drug Application for Dasotraline for the Treatment of Adults with Moderate-to-Severe Binge Eating Disorder