 rivaroxaban
rivaroxaban 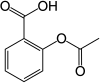 Aspirin
Aspirin rivaroxaban
rivaroxaban  Aspirin
Aspirin"Both heart failure and diabetes are associated with increased blood clotting activity," Abi Khalil said. "Because it decreases platelet aggregation, aspirin is thought to lower the likelihood of forming harmful blood clots like those responsible for heart attacks and strokes."
"Our results suggest that low-dose aspirin therapy in early pregnancy could provide an inexpensive way to lower the preterm birth rate in first-time mothers," said study author Marion Koso-Thomas, M.D., of NICHD's Pregnancy and Perinatology Branch.

"Our results suggest that low-dose aspirin therapy in early pregnancy could provide an inexpensive way to lower the preterm birth rate in first-time mothers," a coauthor said in a statement.https://en.wikipedia.org/wiki/Aspirin
"From this study, we have no evidence to support starting aspirin on day one," Anderson said.
"This study reinforces that," Bozic said.
"The strategy for preventing clots should include medication and early mobilization," he stressed.
 clopidogrel
clopidogrel  aspirin
aspirin"The study gives us solid evidence that we can use this drug combination to prevent strokes in the highest-risk people, but not without some risk of bleeding," said lead author Clay Johnston, M.D., Ph.D., dean and professor of neurology at Dell Medical School at The University of Texas at Austin.
"Of the 33 major hemorrhages that occurred in these 4,881 patients, more than half involved the gastrointestinal tract, and none of them was fatal. These largely preventable or treatable bleeding complications of the treatment have to be balanced against the benefit of avoiding disabling strokes," said co-author J. Donald Easton, M.D., professor of neurology at the University of California, San Francisco School of Medicine.
"The results of this large international trial, when added to the results of previous research, provide evidence to support the use of clopidogrel plus aspirin for 90 days among patients with minor ischemic stroke and high-risk TIA treated within 12 hours," said Ralph Sacco, M.D., M.S., professor of neurology at Miller School of Medicine at the University of Miami. "This trial is likely to change practice since most clinicians and patients are usually willing to accept the increased risk of hemorrhage to offset the disabling impact of a stroke."
"It's likely we will see more patients who have had a TIA or a minor stroke receiving the combination of clopidogrel and aspirin in the future," Johnston said. "If you've suffered from a minor stroke or TIA, it's important to see a physician immediately, even in the emergency room, to ensure you're taking steps to avoid a potentially debilitating stroke later on," he said. "There are several tests that need to be done right away to determine the cause of the event and to make sure the best treatments are started as soon as possible."
Dr. Lesley Stark, of the Cancer Research UK Edinburgh Centre at the University of Edinburgh, said: "We are really excited by these findings as they suggest a mechanism by which aspirin may act to prevent multiple diseases. A better understanding of howaspirin blocks TIF-IA and nucleolar activity provides great promise for the development of new treatments and targeted therapy."
In continuation of my update on aspirin
For adults with type 2 diabetes, low-dose aspirin (ASA) use is associated with a lower risk for myocardial infarction (MI) and stroke, with greater benefit seen with high-frequency use, according to a study presented at the American Heart Association Scientific Sessions 2025, held from Nov. 7 to 10 in New Orleans.
Aleesha Kainat, M.D., from the University of Pittsburgh Medical Center, and colleagues examined the impact of ASA use and adherence frequency on atherosclerotic cardiovascular disease (ASCVD) outcomes among adult patients with diabetes with a moderate or high 10-year ASCVD risk score. Baseline characteristics were balanced between ASA users and nonusers in a propensity score-matched analysis.
Among 11,618 patients, 88.6 and 53.15 percent were ASA and statin users, respectively, at any point during 10-year follow-up. The researchers found that the cumulative incidence of MI, stroke, and 10-year all-cause mortality was significantly lower in the ASA group versus the no-ASA group (42.4 versus 61.2 percent, 14.5 versus 24.8 percent, and 33 versus 50.7 percent, respectively). Compared with no ASA use, any ASA use was associated with significantly lower hazards of MI and ischemic stroke, with greater benefit seen in the high-frequency use group (hazard ratios, 0.54 and 0.47, respectively). Consistent benefit was seen for ASA across glycemic strata, although the magnitude of benefit decreased with worse glycemic control. In better controlled groups, the reduction in mortality was also more pronounced.
"We were somewhat surprised by the magnitude of the findings," Kainat said in a statement. "People with type 2 diabetes and a higher risk of CVD who reported taking low-dose aspirin were much less likely to have had a heart attack, stroke, or death over 10 years when compared to similar individuals who did not report taking low-dose aspirin. That benefit was greatest for those who took aspirin consistently, throughout most of the follow-up time
"That's what makes aspirin so scientifically and clinically interesting," says Chris Paraskeva at the University of Bristol, UK, who was not involved in the work. "It potentially works through a number of different pathways."
The main finding was that the active medication, used in a low dose once a day, successfully reduced spikes in blood pressure and heart rate, as well as demonstrating some positive change in blood clotting tendency."Professor Geoffrey Tofler, lead investigator

This is an important study because it shows ways to improve the physical and mental health of at-risk bereaved people. It is a preventive intervention that is potentially practice-changing, using inexpensive, commonly available medicines."