Certain ingredients in essential oils made from plants such as cloves, anise, fennel and ylang-ylang could serve as a natural treatment of lung and liver conditions caused by air pollution. This is according to Miriana Kfoury of the Unité de Chimie Environnementale et Interactions sur le Vivant, Université du Littoral Côte d'Opale in France and the Lebanese University in Lebanon. She is the lead author of a study in Springer's journal Environmental Chemistry Letters. It is the first of its kind to evaluate the value of using certain essential oil compounds to treat inflammation caused by the fine particles that are typical of hazy, polluted air, and that are known to be carcinogenic.

[The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acid phenylalanine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of cinnamic acid, which is synthesized from phenylalanine in the first step of phenylpropanoid biosynthesis. Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogens, and mediate plant-pollinator interactions as floral pigments and scent compounds. Concentrations of phenylpropanoids within plants are also altered by changes in resource availability].
 Isoeugenol
Isoeugenol 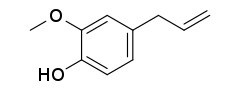 Eugenol
Eugenol
 Estragole
Estragole 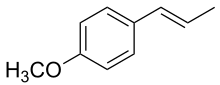 Anethole
Anethole
 Isoeugenol
Isoeugenol  Eugenol
Eugenol Estragole
Estragole  Anethole
Anethole
Plants naturally contain various essential oils that are made up of different compounds. Some of these have been found to have antioxidant value, and to also be able to fight inflammation. A group of organic compounds called phenylpropanoids are found in the essential oils of some plants, and show promise as possible anti-inflammatory substances. Among these are trans-anethole (a flavor component of anise and fennel), estragole (found in basil), eugenol (which occurs in clove bud oil) and isoeugenol (contained in ylang ylang).
Kfoury and her collaborators first collected air pollutant samples containing fine particles in Beirut, Lebanon. In laboratory tests, the samples were then introduced to human cell cultures of normal bronchial epithelial cells (BEAS-2B) and cancer derived hepatic cells (HepG2). The fine particle matter was found to induce inflammation in the cells - these started to secrete the pro-inflammatory cytokines IL-6 and IL-8 (substances that are secreted during infections and tissue damage). Cytokin levels normally increase when the body's immune system is fighting a specific infection.
Next, the researchers established that the trans-anethole, estragole, eugenol and isoeugenol all have so-called cytotoxicity, which means that they could cause cell death at relatively high concentrations. In this evaluation, they were able to determine the level of cytotoxicity of these oil compounds. This was important in order to establish the maximum dose to be selected in the next step, namely the assessment for anti-inflammatory properties. In the second round of tests, the four compounds were introduced to the combination of cell lines and air pollutants to see whether these could protect liver and lung cells damaged by fine particle air pollutants. It was found that the essential oil compounds tested decrease the levels of the two types of cytokines in the samples. The levels of cytokine IL-6 decreased up to 96 percent, and the levels of cytokine IL-8 by 87 percent.
"The findings provide the first evidence that natural essential oil components counteract the inflammatory effects of particulate matter, such as that contained in polluted air," says Kfoury.