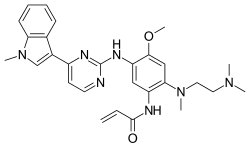
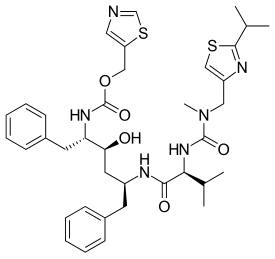
In continuation of my update on Naltrexone
Valeant Pharmaceuticals International, Inc. and Progenics Pharmaceuticals, Inc. today announced that the U.S. Food and Drug Administration has approved Relistor (methylnaltrexone bromide) Tablets for the treatment of opioid-induced constipation (OIC) in adults with chronic non-cancer pain. Valeant expects to commence sales of Relistor Tablets in the U.S. in the third quarter of 2016.

"Opioid-induced constipation represents a long-lasting and potentially debilitating side effect of opioid therapy for millions of patients suffering from chronic pain," commented Joseph C. Papa, Chief Executive Officer of Valeant. "We believe Oral Relistor represents a new alternative treatment for OIC, and we look forward to introducing the more convenient oral formulation as soon as practicable."
"We are delighted that this milestone for Relistor has been achieved, and that patients suffering from OIC will have this new treatment option," said Mark Baker, Chief Executive Officer of Progenics. "We expect the market to be receptive to a more convenient oral tablet formulation of Relistor's well-established subcutaneous preparation. We would like to thank, in particular, Dr. Tage Ramakrishna and Dr. Robert Israel of Valeant for their work over many years in the clinical development of Relistor."
"Relistor has a unique mechanism of action that binds to mu-opioid receptors without impacting the opioid-mediated analgesic effects on the central nervous system," said Richard L. Rauck, MD, Medical Director, Center for Clinical Research, President, Carolinas Pain Institute, President of the Sceptor Pain Foundation of which he is a founding member, and Immediate Past President of the World Institute of Pain. "This represents a true breakthrough in the treatment of OIC, and addresses a large and growing need in the field of pain management." Today, the FDA approved Relistor Tablets (450 mg once daily) for the treatment of OIC in adults with chronic non-cancer pain. Previously, Relistor Subcutaneous Injection (12 mg and 8 mg) was approved in 2008 for the treatment of OIC in adults with advanced illness who are receiving palliative care and in 2014 for the treatment of OIC in adults with chronic non-cancer pain. About the Phase 3 Clinical Trial of Oral Relistor for OIC in Chronic Non-Cancer Pain (NCP) A randomized, double-blind, Phase 3 trial was conducted to evaluate once-daily dosing of 450 mg (n=200) methylnaltrexone (MNTX) tablets compared to placebo (n=201) in adults with chronic NCP. In the 450 mg treatment arm, MNTX tablets demonstrated statistically significant improvements in rescue-free bowel movement (RFBM) within 4 hours of administration over 28 days of dosing when compared to placebo treatment, achieving the primary endpoint. The 450 mg treatment group also achieved statistical significance for the first key secondary efficacy endpoint where a higher percentage of responders (i.e., had ≥3 RFBMs/week, with an increase of ≥1 RFBM/week from baseline for at least 3 of the 4 weeks) was observed with MNTX treatment as compared to placebo. Overall, efficacy of oral methylnaltrexone in this study was comparable to that reported in clinical studies of subcutaneous methylnaltrexone in subjects with chronic, non-cancer pain. The overall observed safety profile seen in patients treated with oral methylnaltrexone was comparable to placebo in this study.
Important Safety Information
Relistor (methylnaltrexone bromide) Tablets is contraindicated in patients with known or suspected gastrointestinal obstruction and patients at increased risk of recurrent obstruction, due to the potential for gastrointestinal perforation.
Cases of gastrointestinal perforation have been reported in adult patients with OIC and advanced illness with conditions that may be associated with localized or diffuse reduction of structural integrity in the wall of the gastrointestinal tract (e.g., peptic ulcer disease, Ogilvie's syndrome, diverticular disease, infiltrative gastrointestinal tract malignancies or peritoneal metastases). Take into account the overall risk-benefit profile when using Relistor in patients with these conditions or other conditions which might result in impaired integrity of the gastrointestinal tract wall (e.g., Crohn's disease). Monitor for the development of severe, persistent, or worsening abdominal pain; discontinue Relistor in patients who develop this symptom.
If severe or persistent diarrhea occurs during treatment, advise patients to discontinue therapy with Relistor and consult their healthcare provider.
Symptoms consistent with opioid withdrawal, including hyperhidrosis, chills, diarrhea, abdominal pain, anxiety, and yawning have occurred in patients treated with Relistor. Patients having disruptions to the blood-brain barrier may be at increased risk for opioid withdrawal and/or reduced analgesia. Take into account the overall risk-benefit profile when using Relistor in such patients. Monitor for adequacy of analgesia and symptoms of opioid withdrawal in such patients.
Avoid concomitant use of Relistor with other opioid antagonists because of the potential for additive effects of opioid receptor antagonism and increased risk of opioid withdrawal. The most common adverse reactions (≥ 12%) in adult patients with opioid-induced constipation and chronic non-cancer pain receiving Relistor tablets were abdominal pain, diarrhea, headaches, abdominal distention, hyperhidrosis, anxiety, muscle spasms, rhinorrhea, and chills. Adverse reactions of abdominal pain, diarrhea, hyperhidrosis, anxiety, rhinorrhea, and chills may reflect symptoms of opioid withdrawal.
About Relistor
Progenics has exclusively licensed development and commercialization rights for its first commercial product, Relistor, to Valeant. Relistor Tablets (450 mg once daily) is approved in the United States for the treatment of OIC in patients with chronic non-cancer pain. Relistor Subcutaneous Injection (12 mg and 8 mg) is a treatment for opioid-induced constipation approved in the United States and worldwide for patients with advanced illness and chronic non-cancer pain.


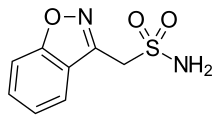
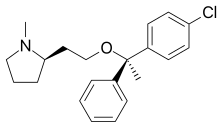
 Dabigatran
Dabigatran Rivaroxaban
Rivaroxaban Apixaban
Apixaban  Edoxaban
Edoxaban