Alkermes plc (Nasdaq: ALKS) announced that the U.S. Food and Drug Administration (FDA) has accepted for review the company's New Drug Application (NDA) seeking approval of ALKS 3831 (olanzapine/samidorphan) for the treatment of schizophrenia and for the treatment of bipolar I disorder. ALKS 3831 is an investigational, novel, once-daily, oral atypical antipsychotic drug candidate designed to provide the efficacy of olanzapine while mitigating olanzapine-associated weight gain. The NDA has been assigned a Prescription Drug User Fee Act (PDUFA) target action date of Nov. 15, 2020. [(Olanzapine/samidorphan - developmental code name ALKS-3831) is a combination of the atypical antipsychotic olanzapine and the opioid modulator samidorphan
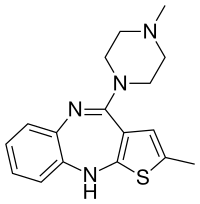
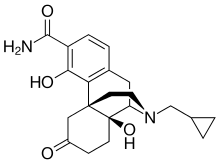
Samidorphan
olanzapine
"The acceptance of the NDA for ALKS 3831 marks an important milestone toward our goal of offering a new treatment option to people living with schizophrenia or bipolar I disorder. The ALKS 3831 development program builds on Alkermes' commitment to developing new therapeutic options that seek to address unmet needs of patients in large therapeutic areas," said Craig Hopkinson, M.D., Chief Medical Officer at Alkermes. "We believe ALKS 3831 has the potential to be a meaningful new offering for patients with these serious and complex mental health disorders, and we look forward to engaging with the FDA throughout the NDA review process."
The ALKS 3831 NDA includes data from the ENLIGHTEN clinical development program in patients with schizophrenia, as well as pharmacokinetic (PK) bridging data comparing ALKS 3831 and ZYPREXA® (olanzapine), to support an indication for the treatment of schizophrenia, and an indication for the treatment of manic or mixed episodes associated with bipolar I disorder as a monotherapy or adjunct to lithium or valproate and for maintenance treatment of bipolar I disorder. Alkermes is seeking approval of fixed dosage strengths of ALKS 3831 composed of 10 mg of samidorphan co-formulated with 5 mg, 10 mg, 15 mg or 20 mg of olanzapine.
About the ENLIGHTEN Clinical Development Program
The ENLIGHTEN clinical development program for ALKS 3831 includes two key studies in patients with schizophrenia: the ENLIGHTEN-1 study, which evaluated the antipsychotic efficacy of ALKS 3831 compared to placebo over four weeks, and the ENLIGHTEN-2 study, which assessed weight gain with ALKS 3831 compared to olanzapine over six months. The program also includes supportive studies to evaluate the pharmacokinetic and metabolic profile and long-term safety of ALKS 3831, and pharmacokinetic bridging studies comparing ALKS 3831 and ZYPREXA.
About ALKS 3831
ALKS 3831 is an investigational, novel, once-daily, oral atypical antipsychotic drug candidate for the treatment of schizophrenia and bipolar I disorder. ALKS 3831 is composed of samidorphan, a novel, new molecular entity, co-formulated with the established antipsychotic agent, olanzapine, in a single bilayer tablet.
About Schizophrenia
Schizophrenia is a serious brain disorder marked by positive symptoms (hallucinations and delusions, disorganized speech and thoughts, and agitated or repeated movements) and negative symptoms (depression, blunted emotions and social withdrawal).An estimated 2.4 million American adults have schizophrenia, with men and women affected equally.
About Bipolar I Disorder
Bipolar disorder is a brain disorder that causes shifts in a person's mood, energy and ability to function. Patients with this brain disorder may experience debilitating mood shifts from extreme highs (mania) to extreme lows (depression). Bipolar I disorder is characterized by the occurrence of at least one manic episode, with or without the occurrence of a major depressive episode, and affects approximately one percent of the adult population in the United States in any given year.
Alkermes plc is a fully integrated, global biopharmaceutical company developing innovative medicines for the treatment of central nervous system (CNS) diseases and oncology. The company has a diversified commercial product portfolio and a clinical pipeline of product candidates for diseases that include schizophrenia, depression, addiction, multiple sclerosis, and cancer. Headquartered in Dublin, Ireland, Alkermes plc has an R&D center in Waltham, Massachusetts; a research and manufacturing facility in Athlone, Ireland; and a manufacturing facility in Wilmington, Ohio. For more information, please visit Alkermes' website at www.alkermes.com.
https://en.wikipedia.org/wiki/Olanzapine
https://en.wikipedia.org/wiki/Samidorphan
