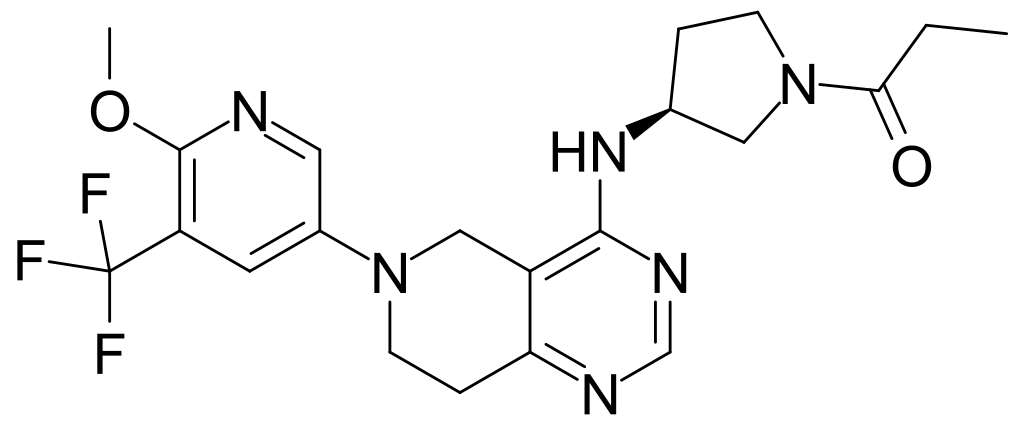
Pharming Group N.V. ("Pharming" or "the Company") (EURONEXT Amsterdam: PHARM/Nasdaq: PHAR) announced the USFDA approval of Joenja (leniolisib) for the treatment of activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS) in adult and pediatric patients 12 years of age and older. Joenja®, an oral, selective PI3Kδ inhibitor, is the first and only treatment approved in the US for APDS, a rare and progressive primary immunodeficiency. The FDA evaluated the Joenja® application for APDS under Priority Review, which is granted to therapies that have the potential to provide significant improvements in the treatment, diagnosis or prevention of serious conditions. Joenja® is expected to launch in the US in early April and Dr. Eveline Wu, MD, MSCR, Division Chief, Paediatric Rheumatology & Associate Professor of Paediatric Rheumatology and Allergy/Immunology at The University of North Carolina School of Medicine, said:
"The FDA approval of Joenja® is an exciting moment for the APDS community and offers to transform the treatment pathway for patients and families affected by this rare disease. This approval means that they will, for the first time, have access to an approved treatment, which has the potential to change the standard of care for the patient population suffering from APDS."
Vicki Modell, co-founder of the Jeffrey Modell Foundation, an international, non-profit, organization dedicated to helping individuals and family members affected by primary immunodeficiency disorders, commented:
"The approval of Pharming's Joenja® is an important step toward making a difference in the lives of individuals living with APDS who experience severe, life-altering and progressive symptoms. The FDA approval of a treatment option for one of the more than 450 primary immunodeficiencies is also a key moment for the broader primary immunodeficiency community. The Jeffrey Modell Foundation's mission of hope, advocacy and action is dedicated to early diagnosis, genetic sequencing, treatments and ultimately, future cures for primary immunodeficiencies."
Sijmen de Vries, Chief Executive Officer of Pharming, commented:
"This FDA approval of Joenja® is an important milestone for people living with APDS who will now have access to the first approved treatment option specifically for this debilitating disease. Until now, management of APDS has relied on the treatment of the diverse symptoms associated with APDS. We are grateful to the patients, caregivers, and physicians who participated in the clinical trials who have made today's approval a reality. I would also like to thank the Pharming and the Novartis teams who have supported the development of Joenja® and can, therefore, be justifiably proud of this FDA approval.
Today also marks a landmark event for Pharming and demonstrates our commitment to transforming the lives of patients who suffer from rare diseases. The approval and near-term launch of Joenja®, our second commercial product, brings us closer to our goal of becoming a leading global rare disease company dedicated to patient communities with unmet medical needs."
APDS is a rare primary immunodeficiency that was first characterized in 2013 and is currently estimated to affect 1 to 2 people per million. It is caused by genetic variants in either one of two identified genes, known as PIK3CD or PIK3R1, which are vital to the normal development and function of immune cells in the body. While people with APDS may suffer from a wide variety of symptoms, the most common are frequent and severe infections of the ears, sinuses, and upper and lower respiratory tracts. Infections usually begin in infancy. People with APDS are susceptible to swollen lymph nodes or an enlarged spleen (splenomegaly), as well as autoimmunity and inflammatory symptoms. People with APDS may also be at higher risk for cancers like lymphoma.
https://en.wikipedia.org/wiki/Leniolisib