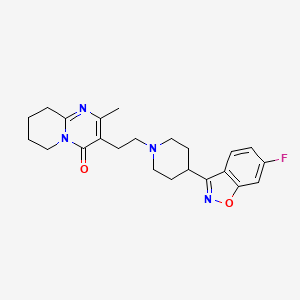
Teva Pharmaceuticals, announced the U.S. Food and Drug Administration (FDA) approval of Uzedy (risperidone) extended-release injectable suspension for the treatment of schizophrenia in adults. Uzedy is the first subcutaneous, long-acting formulation of risperidone that utilizes SteadyTeq™, a copolymer technology proprietary to MedinCell that controls the steady release of risperidone. Therapeutic blood concentrations are reached within 6-24 hours of a single dose.
“Uzedy embodies Teva’s commitment to bringing innovative advances to patients and to providing people living with schizophrenia an important new treatment option that was designed to address certain treatment challenges and may decrease the risk of relapse,” said Richard Francis, President and CEO of Teva. “The approval of Uzedy is a culmination of a multidisciplinary effort across Teva and MedinCell to bring this important treatment to market. This milestone is a testament to advancing our robust biopharmaceutical pipeline of innovative medicines that aim to support more people living with mental health disorders and neurological diseases in the coming years.”
Approximately 80% of patients with schizophrenia experience multiple relapses over the first five years of treatment,2 most commonly due to suboptimal adherence to treatment with oral antipsychotics. Each relapse carries a biological risk of loss of function, treatment refractoriness, and changes in brain morphology.3,4
Schizophrenia is a chronic, progressive and severely debilitating mental health disorder that affects how one thinks, feels and acts.5 This approval is based on data from two Phase 3 trials evaluating Uzedy in patients with schizophrenia: TV46000-CNS-30072 (the RISE Study – The Risperidone Subcutaneous Extended-Release Study) and TV46000-CNS-30078 (the SHINE Study – A Study to Test TV-46000 for Maintenance Treatment of Schizophrenia).
“The approval of the first product formulated with our technology is a pivotal moment for MedinCell and for the many patients who will benefit,” said Christophe Douat, CEO of MedinCell. “We are committed to supporting patients through innovative therapy options. It continues to be a wonderful journey with Teva, an ideal partner to harness the full potential of Uzedy. Our technology reaching commercial stage marks the start of an exciting new era for MedinCell and we are extremely proud to share this very special moment with all our employees and shareholders.”
The use of novel SteadyTeq technology in Uzedy controls the release of risperidone over time. The initiation of treatment requires no loading dose or oral supplementation. Therapeutic blood concentrations are reached within 6-24 hours of a single dose.
“Treatments for schizophrenia are largely prescribed as daily oral medications, which can present challenges with adherence due to missed doses. Lack of adherence to treatment with oral antipsychotics is the most common cause of relapse in schizophrenia so there’s a role for therapies that are dosed in one- or two-month dosing intervals to help prevent relapse,” said Christoph Correll, MD, professor of psychiatry at the Zucker School of Medicine, Hempstead, NY. “As a clinician, I am excited to now have a new treatment option that reduces the risk of relapse1 for this complex disease and helps address some of the barriers around receiving schizophrenia treatment.”