A team of scientists at the University of Nebraska Medical Center (UNMC) and Longevity Biotech, Inc., has demonstrated that neuroprotection could be attained in preclinical models by a novel drug candidate that changes immune responses.
The results, published today in the Journal of Neuroscience, describe the prevention of nerve cell damage in a mouse model of Parkinson's disease. Notably, the drug protected nerve cells that produce dopamine, which is the chemical responsible for agility and movement that is lost in human disease.
"The results are exciting as they provide a bridge between the immune system and nerve cell protection in Parkinson's disease," said Scott Shandler, Ph.D., co-founder and CEO of Longevity Biotech.
"The idea was birthed nearly a decade ago when specific types of circulating blood cells called lymphocytes were found to damage the types of nerve cells responsible for disease," said Howard Gendelman, M.D., the Margaret R. Larson Professor and chair of the UNMC Department of Pharmacology and Experimental Neuroscience. "The new Longevity Biotech drug (LBT-3627) was able to change the function of these cells from killing the nerve cells to protecting them. This is especially significant for the Nebraska team, as the mechanism parallels closely the human trials nearing completion for Parkinson's patients."
LBT-3627 is similar to the naturally occurring vasoactive intestinal peptide (VIP), a well-established anti-inflammatory peptide with beneficial effects across a variety of disorders. VIP is rapidly degraded by the body and is unable to distinguish between its two naturally intended receptors (VPAC1 vs. VPAC2). These limitations have stymied prior translational success using VIP.


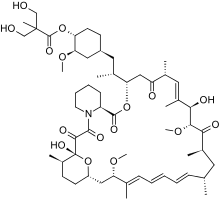 Temsirolimus
Temsirolimus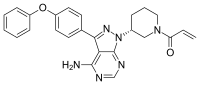 Ibrutinib
Ibrutinib

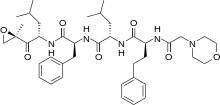
 Crizotinib
Crizotinib