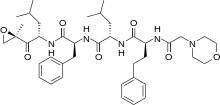
In continuation of my update on carfilzomib
Carfilzomib significantly improves outcomes in previously treated patients with relapsed or refractory multiple myeloma, shows a head-to-head comparison with bortezomib.
In the ENDEAVOR phase III trial, published in The Lancet Oncology, median progression-free survival (PFS) was 18.7 months for the 464 patients randomly assigned to receive open-label carfilzomib plus dexamethasone. This was significantly longer than the 9.4 months for the 465 participants treated with bortezomib and dexamethasone, and equated to a 47% risk reduction in favour of carfilzomib.
Moreover, a significantly higher proportion of carfilzomib- than bortezomib-treated patients achieved an objective response, at 77% versus 63%, and the duration of response was also longer in the former group, at a respective 21.3 and 10.4 months.
The most common side effects of grade 3 or worse that occurred more frequently in the carfilzomib than the bortezomib treatment arm were anaemia and hypertension, with rates of 14% versus 10% and 9% versus 3%, respectively.
However, peripheral neuropathy of grade 3 was observed in 2% of carfilzomib-treated patients and there were no grade 4 events, compared with 8% of patients in the bortezomib arm who experienced events of grade 3 or 4.
Serious adverse events occurred in 48% of patients in the carfilzomib group and in 36% of those given bortezomib, but Meletios Dimopoulos (National and Kapodistrian University of Athens, Greece) and team note that the number of discontinuations and deaths attributable to adverse events were comparable between the groups.
They conclude that carfilzomib plus dexamethasone could be considered for multiple myeloma patients for whom bortezomib is indicated.
