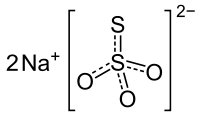
Tuesday, August 22, 2017
New treatment prevents chemotherapy-induced hearing loss in children with cancer

Tuesday, February 23, 2016
Cancer drug may protect kidneys from damage caused by chemotherapy agent cisplatin

Sunday, December 27, 2009
Mitaplatin as a better anticancer agent.......
As per the claim by the authors, the negative intracellular redox potential reduces the platinum to release cisplatin, a Pt (II) compound, and two equivalents of DCA. By a unique mechanism, mitaplatin thereby attacks both nuclear DNA with cisplatin and mitochondria with DCA selectively in cancer cells. The cytotoxicity of mitaplatin in a variety of cancer cell lines equals or exceeds that of all known Pt (IV) compounds and is comparable to that of cisplatin.
Mitaplatin alters the mitochondrial membrane potential gradient of cancer cells, promoting apoptosis by releasing cytochrome c and translocating apoptosis inducing factor from mitochondria to the nucleus. Cisplatin formed upon cellular reduction of mitaplatin enters the nucleus and targets DNA to form 1,2-intrastrand d(GpG) cross-links characteristic of its own potency as an anticancer drug. These properties of mitaplatin are manifest in its ability to selectively kill cancer cells cocultured with normal fibroblasts and to partially overcome cisplatin resistance. Further studies like mice transplanted with human tissues are to be substantiated, in my opinion its a good achievement...
Ref : http://www.pnas.org/content/early/2009/12/09/0912276106.abstract?related-urls=yes&legid=pnas;0912276106v1
Friday, February 6, 2015
Addition of S-1 to cisplatin plus radiotherapy ‘favourable’ in NSCLC
Friday, October 10, 2014
Phase III trial: Rolapitant lessens chemotherapy-induced nausea and vomiting
Saturday, June 13, 2009
Cisplatin doubles lung cancer survival time in mice !
After so many years, I could find this something interesting findings about cisplatin, by Patrizia Russo of Lung Cancer Unit of the National Cancer Research Institute in Genoa, Italy and colleagues from San Raffaele Pisana Scientific Institute for Research, Hospitalization and Health Care (IRCCS), Catholic University.
In the study, the authors took the research a step further and showed that α-CbT could inhibit non-small cell lung carcinoma (NSCLC) growth and prolong life in non-obese/severe combined immunodeficient (NOD/SCID) mice that had human NSCLC grafted to their lungs. This study attempted to mimic human cancer conditions more closely by delaying treatment until the tumors were well-established. In addition to control mice that were untreated, the researchers randomized one third of the mice to receive standard chemotherapy.
They found that NOD/SCID mice treated with the standard chemotherapy agent, cisplatin, had a 16 percent longer median survival time than untreated mice (p= 0.05). Mice treated with α-CbT, however, had an increased median survival time of 1.7-fold over the cisplatin-treated mice and 2.1-fold over the no-treatment controls (p=0.0005). Though the clinical trials to establish the claim and to to explore the widest range of possibilities of intervention on the α7-nAChRs. Congrats...
Ref :Inhibition of Nonneuronal 7-Nicotinic Receptor for Lung Cancer Treatment; Am. J. Respir. Crit. Care Med., Jun 2009; 179: 1141 - 1150
Tuesday, March 31, 2020
One-Cycle BE500P Seems Safe for High-Risk Early Testicular Cancer
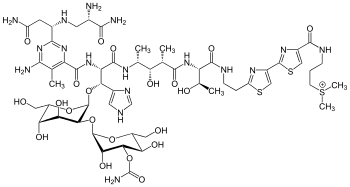


https://en.wikipedia.org/wiki/Bleomycin
https://en.wikipedia.org/wiki/Etoposide
https://en.wikipedia.org/wiki/Cisplatin
Friday, July 29, 2016
Added benefit of crizotinib drug for first-line treatment of advanced bronchial carcinoma not proven

Friday, March 19, 2010
Gemcitabine and cisplatin a promising combination for endometrial cancer...
Gemcitabine (see structure) and cisplatin in combination have been investigated extensively in other disease sites, and synergism of the two agents has been confirmed in cell lines of human endometrial, ovarian, colon, lung and squamous cell head and neck carcinoma
The Phase II study of 20 patients found that the combination of gemcitabine and cisplatin, two drugs currently used to treat other types of cancer, limited the disease's progression, increasing progression-free survival while maintaining tolerable toxicity levels. It is believed that when administered together, gemcitabine helps overcome cell resistance to cisplatin, throwing tumor cells a potent one-two punch.
Findings demonstrated a 50 percent overall response rate, or improvement in disease. Additionally, the clinical benefit of the two-drug combination was 80 percent, as 16 of the 20 women experienced either an improvement or stabilization of disease. All side effects resulting from the therapy were manageable. Lead researcher, Dr. Brown concluded that results from the study warrant investigation of the chemotherapy combination in a larger, definitive trial at multiple institutions.....
Ref : Dr. Jubilee Brown, http://www.mdanderson.org/
Monday, December 26, 2016
Drug candidate delivered by plant-virus-based carrier shows promise for triple-negative breast cancer
 phenanthriplatin
phenanthriplatinThursday, October 21, 2010
Turmeric component (curcumin) enhances chemotherapy's suppression of head and neck cancer
In continuation of my update on Curcumin, I found this info interesting to share with.., i.e., researchers with UCLA's Jonsson Cancer Center have found, when combined with the drug Cisplatin, turmeric enhances the chemotherapy's suppression of head and neck cancer cell growth. Previous studies have shown it can suppress the growth of certain cancers. The study, done in cells in Petri dishes and then in mouse models is of great importance.
A 2005 study by Wang and Srivatsan first showed that curcumin suppressed the growth of head and neck cancer cells, first in cells and then in mouse models. In the animal studies, the curcumin was applied directly onto the tumors in paste form because it did not dissolve in saline, which would have allowed it to be injected. n need of a better way to deliver the curcumin, the team collaborated with Dr. Kapil Mehta of M.D. Anderson Cancer Center and found that encapsulating the curcumin in a liposome, an artificially prepared vehicle that enclosed the spice component within its membrane, made the treatment injectable. The curcumin was injected into the tail vein of a mouse, where it circulated into the blood stream, slowing down and eventually stopping the cancer growth, a study in 2008 found.
"This was a very positive finding, developing an efficient way to deliver the treatment," Wang said. "Our study also showed that the curcumin was very well tolerated."
In this study, the team wanted to combine the curcumin with the chemotherapeutic drug Cisplatin, which is very toxic at the doses needed to fight head and neck cancers, damaging kidneys, the ears and the bone marrow. They hoped that if they added curcumin to the mix, they might be able to lower the Cisplatin dose and cause less organ damage. Their finding, that the curcumin made the Cisplatin work better, was very promising.
More....
Saturday, September 22, 2012
New drug candidate shows promise against cancer - MIT Media Relations
“I’ve long believed that there’s something special about platinum and its ability to treat cancer. Using new variants, we might have a chance of applying platinum to a broader range of cancer types, more successfully,” said Lippard. Lippard is senior author of a paper describing the new drug candidate, known as phenanthriplatin - which is cis-[Pt(NH3)2(phenanthridine)Cl]NO3.
Monday, October 14, 2013
Active ingredient of ipecac syrup inhibits growth of cancer cells
- Emetine alone inhibits the proliferation of bladder cancer cell lines.
- Emetine acts synergistically with cisplatin to inhibit bladder cancer proliferation better than either drug does alone.
- Emetine has little effect on normal cells.
"There is an urgent need to develop new drug combinations," Dr. Gupta said. "Our study demonstrates that combining emetine with cisplatin is potentially beneficial, and merits further study in clinical trials."
Tuesday, December 20, 2016
Metformin along with chemotherapy/radiation improves outcomes in head and neck cancer patients

"In basic science studies, metformin has been shown to stop mTOR, a molecular pathway present and active in this type of head and neck cancer, and pretreatment with metformin resulted in a decrease in the occurrence of oral cavity tumors in animal models. In this study, we wanted to see if the combination of escalating doses of metformin with the chemotherapy agent cisplatin and radiation for head and neck cancer tumors in non-diabetic patients would be effective."
"This is part of an ongoing clinical trial," says Wise-Draper. "We found that eight patients with advanced head and neck cancer have been enrolled so far; we plan to have 30 total. Due to the relatively quick escalation of metformin, the patients' tolerance was poor with higher doses of metformin when initiated 7 days prior to their chemotherapy and radiation therapy regimen.
"Therefore, the protocol was modified to allow slower escalation over 14 days. The most common toxicities observed included nausea (71 percent of patients) and vomiting (43 percent of patients), increase in creatinine (57 percent of patients), decreased white blood cell count (43 percent of patients) and pain when swallowing (43 percent of patients) with only nausea being directly attributed to metformin and the rest attributed to cisplatin and radiation."
Saturday, November 21, 2009
Picoplatin a better drug than oxaliplatin for colorectal cancer !
About Cis-platin & other drivatives:
Cisplatin, cisplatinum, or cis-diamminedichloroplatinum(II) is a platinum-based chemotherapy drug used to treat various types of cancers, (sarcomas, some carcinomas (small cell lung cancer, and ovarian cancer), lymphomas, and germ cell tumors. It was the first member of a class of anti-cancer drugs which now also includes carboplatin and oxaliplatin. These platinum complexes react in vivo, binding to and causing crosslinking of DNA which ultimately triggers apoptosis (programmed cell death).
Now its the turn of Picoplatin [see structure , Amminedichloro(2-methylpyridine)platinium)], Poniard Pharmaceuticals, Inc. has come up with some interesting results from its Phase 2 trial of picoplatin in patients with metastatic colorectal cancer (CRC). Picoplatin, given once every four weeks in combination with 5-fluorouracil and leucovorin in the FOLPI regimen, has comparable efficacy to oxaliplatin, given in combination with 5-fluorouracil and leucovorin in the modified FOLFOX-6 regimen, as a first-line therapy for CRC, as assessed by one-year survival rate, progression-free survival (PFS) and disease control. The company claims that, (from the updated proof-of-concept Phase 2 safety and efficacy results) picoplatin could be superior to oxaliplatin as a neuropathy-sparing alternative when used in combination as a first-line treatment for metastatic colorectal cancer.
Source : http://investor.poniard.com/ReleaseDetail.cfm?ReleaseID=424813.
Thursday, October 30, 2014
VAL-083 drug compound shows promise against non-small cell lung cancer
Tuesday, February 16, 2010
Triapine with cisplatin a new standard of care for cervical cancer?
Wednesday, November 23, 2011
Cisplatin anti-cancer drug binds pervasively to RNA....
Thursday, October 3, 2013
Cisplatin plus radiotherapy and HDRB proves beneficial in stage IIIB cervical cancer
Wednesday, November 25, 2009
Oncolytics Biotech's REOLYSIN combined with paclitaxel and carboplatin well tolerated for advanced cancers
Oncolytics Biotech's REOLYSIN combined with paclitaxel and carboplatin well tolerated for advanced cancers







