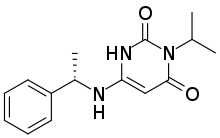
Bristol Myers Squibb (NYSE: BMY) announced the U.S. Food and Drug Administration (FDA) approval of Sotyktu™(deucravacitinib), a first-in-class, oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor, for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Sotyktu is not recommended for use in combination with other potent immunosuppressants.

The approval is based on results from the pivotal Phase 3 POETYK PSO-1 and POETYK PSO-2 clinical trials, which demonstrated superior efficacy of once-daily Sotyktu compared to placebo and twice-daily Otezla® (apremilast) in 1,684 patients aged 18 years and older with moderate-to-severe plaque psoriasis.1 The superior efficacy of Sotyktu compared to placebo and Otezla was demonstrated at both 16 and 24 weeks, and responses with Sotyktu persisted through 52 weeks. See below for more information.
“Sotyktu has the potential to become the new standard of care oral treatment for people with moderate-to-severe plaque psoriasis, given its profile in helping patients achieve clearer skin as demonstrated in the POETYK PSO clinical program,” said April Armstrong, MD, MPH, clinical investigator in the POETYK PSO-1 trial and Associate Dean and Professor of Dermatology at the University of Southern California. “People living with moderate-to-severe plaque psoriasis face significant burdens, and Sotyktu is a welcome first-line systemic treatment option.”
Psoriasis is a widely prevalent, chronic, systemic immune-mediated disease that affects approximately 7.5 million people in the U.S.3 Up to 90 percent of patients with psoriasis have plaque psoriasis, which is characterized by distinct, round or oval plaques typically covered by silvery white scales. Nearly one-quarter of people with psoriasis, or around two million in the U.S., have cases that are considered moderate-to-severe.
“The approval of Sotyktu represents an exciting day for patients suffering from moderate-to-severe plaque psoriasis who are not satisfied with topical and conventional treatments. This is another extraordinary achievement for Bristol Myers Squibb, as we bring forward a new mechanism of action, the first oral treatment approved in nearly 10 years, and the first orally dosed once-daily treatment for moderate-to-severe plaque psoriasis,” said Samit Hirawat, MD, Chief Medical Officer, Bristol Myers Squibb. “We believe Sotyktu is a breakthrough in the treatment of patients with this condition, and we’re excited about its potential in other immune-mediated diseases.”
In the POETYK PSO trials, at Week 16, the most common adverse
reactions (≥1 percent and higher than placebo) in patients on Sotyktu
were upper respiratory infections (19.2 percent), blood creatine
phosphokinase increase (2.7 percent), herpes simplex (2.0 percent),
mouth ulcers (1.9 percent), folliculitis (1.7 percent) and acne (1.4
percent).1 In addition, 2.4 percent of patients on Sotyktu,
3.8 percent of patients on placebo, and 5.2 percent of patients on
Otezla experienced adverse reactions leading to discontinuation.
“Despite the availability of therapies, many people living with plaque psoriasis in the United States are untreated or undertreated,5,6” said Leah M. Howard, JD, President and CEO of the National Psoriasis Foundation. “The FDA approval of a new oral treatment is exciting news for the psoriasis community. We welcome this new treatment option.”