



"Verzenio provides a new targeted treatment option for certain patients with breast cancer who are not responding to treatment, and unlike other drugs in the class, it can be given as a stand-alonetreatment to patients who were previously treated with endocrine therapy and chemotherapy," said Richard Pazdur, M.D., director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.

"The findings in this study show a modest benefit to a subgroup of women with estrogen receptor-positive tumors," said Dr. Stephanie Bernik, a breast cancer specialist who wasn't involved in the research.
"About 40 percent of all patients with advanced breast cancer estrogen receptor-positive have PIK3CA mutations, which means they could benefit from taselisib," explained study author Dr. Jose Baselga. He's physician-in-chief at Memorial Sloan Kettering Cancer Center in New York City.
"Our findings are proof that targeting this pathway in breast cancer is effective. However, the benefit to patients was more modest than we had hoped for, and there is a risk of considerable side effects with the addition of taselisib," Baselga said in a news release from the American Society of Clinical Oncology (ASCO).
"Although tumor growth was only suppressed by two months, this medication opens the door to further investigation with drugs that target cancers with the PIK3CA gene mutation," she said.
"One would hope that because we know targeting this gene decreases tumor growth, perhaps combining it with various other drugs might make it more effective, and also direct research to developing other drugs that work in a similar fashion," Bernik reasoned.
"Compelling data for Kisqali have led to the broadest first-line indications of any CDK4/6 inhibitor," said Liz Barrett, CEO, Novartis Oncology. "With this new approval Kisqali has the potential to help even more people in the US live a longer life without progression of disease from this incurable form of breast cancer."
"These MONALEESA clinical trial program data add to the body of evidence that CDK 4/6 inhibition, in the case of these studies with ribociclib, gives women diagnosed with HR+/HER2- advanced breast cancer an important first-line treatment option," said Dennis J. Slamon, MD, Director of Clinical/Translational Research, University of California, Los Angeles Jonsson Comprehensive Cancer Center. "Based on Phase III trial results that consistently showed clinical benefit, physicians should be encouraged to re-evaluate treatment for advanced breast cancer in the first-line setting."
"Premenopausal women diagnosed with advanced breast cancer often face unique social challenges and a poorer prognosis. For the first time in nearly 20 years, we have results from a dedicated clinical trial among these women," said Jennifer Merschdorf, CEO, Young Survival Coalition. "With this approval, some younger women now have a new therapy indicated specifically for them that may help extend their lives without progression of disease."
“With this approval, we are now able to offer Ibrance to the underserved male breast cancer community and provide more patients with HR+, HER2- metastatic breast cancer the opportunity to access an innovative medicine,” said Chris Boshoff, M.D., Ph.D., Chief Development Officer, Oncology, Pfizer Global Product Development. “We appreciate that our partnership with the FDA has allowed us to take a significant step forward in the use of real-world data to bring medicines to patients who are most in need.”
“Men with breast cancer have limited treatment options, making access to medicines such as Ibrance critically important,” said Bret Miller, founder of the Male Breast Cancer Coalition. “We applaud the use of real-world data, a new approach to drug review, to make Ibrance available to certain men with metastatic breast cancer and help address an unmet need for these patients.”

“Piqray is the first PI3K inhibitor to demonstrate a clinically meaningful benefit in treating patients with this type of breast cancer. The ability to target treatment to a patient’s specific genetic mutation or biomarker is becoming increasingly common in cancer treatment, and companion diagnostic tests assist oncologists in selecting patients who may benefit from these targeted treatments,” said Richard Pazdur, M.D., director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. “For this approval, we employed some of our newer regulatory tools to streamline reviews without compromising the quality of our assessment. This drug is the first novel drug approved under the Real-Time Oncology Review pilot program. We also used the updated Assessment Aid, a multidisciplinary review template that helps focus our written review on critical thinking and consistency and reduces time spent on administrative tasks.”
AstraZeneca’s Truqap (capivasertib) in combination with Faslodex (fulvestrant) has been approved in the US for the treatment of adult patients with hormone receptor (HR)-positive, HER2-negative locally advanced or metastatic breast cancer with one or more biomarker alterations (PIK3CA, AKT1 or PTEN). Eligible patients will have progressed on at least one endocrine-based regimen in the metastatic setting or experienced recurrence on or within 12 months of completing adjuvant therapy.
The approval by the Food and Drug Administration (FDA) was based on the results from the CAPItello-291 Phase III trial published earlier this year in The New England Journal of Medicine.1 In the trial, Truqap in combination with Faslodex reduced the risk of disease progression or death by 50% versus Faslodex alone in patients with tumours harbouring PI3K/AKT pathway biomarker alterations (based on hazard ratio of 0.50, 95% confidence interval 0.38-0.65; p=<0.001; median progression-free survival (PFS) 7.3 versus 3.1 months).
Breast cancer is the most common cancer and one of the leading causes of cancer-related death worldwide.2 HR-positive breast cancer (expressing estrogen or progesterone receptors, or both), is the most common subtype, with more than 65% of tumours considered HR-positive and HER2-low or HER2-negative.3 Collectively, mutations in PIK3CA, AKT1 and alterations in PTEN occur frequently, affecting up to 50% of patients with advanced HR-positive breast cancer.4-6 Endocrine therapies are widely used in this setting, but many patients develop resistance to 1st-line cyclin-dependent kinase (CDK) 4/6 inhibitors and estrogen receptor-targeting therapies, underscoring the need for additional endocrine therapy-based options
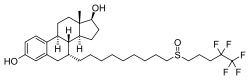
 abemaciclib
abemaciclib
 Palbociclib (codenamed PD-0332991, trade name Ibrance)
Palbociclib (codenamed PD-0332991, trade name Ibrance)
"This approval is an important milestone, as it shows that Verzenio plus an aromatase inhibitor substantially reduced tumor size and delayed disease progression in women with HR+, HER2- metastatic breast cancer. Notably, the MONARCH 3 trial included patients with certain concerning clinical characteristics, such as a pattern of disease that spread to the liver," said Joyce O'Shaughnessy, M.D., Celebrating Women Chair in Breast Cancer Research and chair, Breast Cancer Research Program, Baylor University Medical Center, Texas Oncology and U.S. Oncology, Dallas, TX. "This information will help inform treatment decisions for each patient, which can be complicated in advanced breast cancer."
"The speed with which our team has been able to work with the FDA to gain approval for this additional Verzenio indication underscores Lilly's commitment to delivering meaningful medicines that can help more people living with advanced breast cancer," said Sue Mahony, Ph.D., senior vice president and president of Lilly Oncology. "Verzenio has now been developed, studied and clinically proven in three key trials to be effective for women with HR+, HER2- metastatic breast cancer – helping to ensure we are providing support to those who need it most."