
In the 'Journal of Clinical Investigation', scientists at the Helmholtz Zentrum München describe a small peptide that very efficiently binds excess copper from liver cells. This molecule comes from a bacterium's bag of tricks and could be suitable for treating Wilson disease. In an experimental model it has already proven superior to conventional medicines.
In Wilson disease, also called Wilson's disease or hepatolenticular degeneration, the body is no longer able to excrete excess copper ingested from food into the intestines via the bile. Instead, the copper is stored in the liver and other organs, where it can cause severe damage. Doctors accordingly employ medicines called chelators that bind the surplus copper. These life-long treatments are especially effective if commenced during the early stages of the disease. The drugs must be taken several times a day, are repeatedly associated with undesired effects, and, particularly in the event of a late diagnosis of the disease, are often ineffective, so that a liver transplant can be necessary as the last resort.
Researchers headed by PD Dr. Hans Zischka, head of the Oxidative Cell Death research group at the Institute of Molecular Toxicology and Pharmacology at the Helmholtz Zentrum München have now conducted a detailed examination of a bacterial agent that could improve the disease treatment. They looked to the bacterium Methylosinus trichosporium, which requires large quantities of copper due to its special methane metabolism. In order to acquire the necessary metal, it excretes the methanobactin molecule, which very efficiently binds copper.
In order to check if methanobactin is also suitable for binding copper from the body, the researchers used an in vivo model for the disease that had the same genetic defect as that found in humans. "We were able to observe that even acute stages of Wilson disease reversed with methanobactin," reports Josef Lichtmannegger, who, together with Christin Leitzinger, is the study's first author. Further analyses showed that the improvement was due to a sharp decline in the copper quantities. Especially the mitochondria, known as the "powerhouse of the cell", greatly profited from the dropping copper levels and were able to resume their full function. Methanobactin hindered the death of liver cells and prevented liver failure.
The researchers then compared methanobactin to chelators that are currently used in hospitals. Unlike the chelators, methanobactin was able to eliminate the copper overload in the liver cells within a few days, even in stages of severe damage, and prevent organ failure. The agent was also very well tolerated in the model.
"We hope that our work will make it possible to improve the treatment of Wilson disease and reduce the number of liver transplants," states Zischka, the study leader. It is conceivable that in the long run it will be possible to replace the current use of less effective chelators several times a day with short treatment cycles using methanobactin. Clinical studies are now necessary to test this.



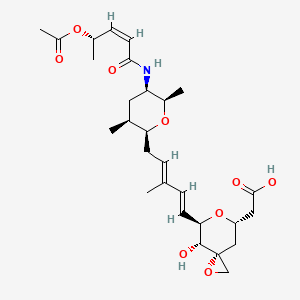


 carfilzomib
carfilzomib
 empagliflozin
empagliflozin  In continuation of my update on
In continuation of my update on 