Richard B. Lipton, MD, Professor of Neurology and Director of the Montefiore Headache Center, Albert Einstein College of Medicine, commented, “A significant proportion of migraine patients experience inadequate efficacy with currently available acute treatments, leading to even greater suffering, and an increased risk of worsening of migraine pain and attack frequency. Results of multiple clinical trials demonstrate that Symbravo can provide rapid and long-lasting freedom from migraine pain, whether treatment is taken early in the attack while the pain is mild, or later in the attack when the pain may be severe. The approval of Symbravo is a long awaited and much welcomed advancement for clinicians and our patients, providing a new, meaningful treatment option.”
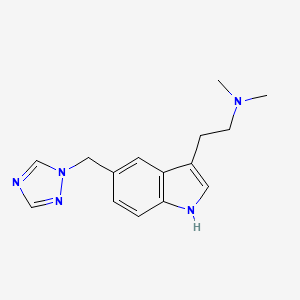
Rizatriptan
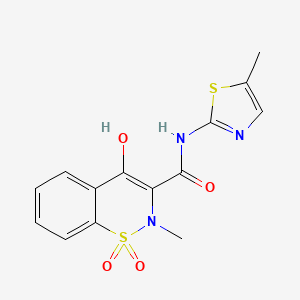
meloxicam
Stewart Tepper, MD, Clinical Professor of Neurology at the Geisel School of Medicine at Dartmouth and Vice President of the New England Institute for Neurology and Headache, said, “Migraine is a debilitating condition that affects millions of Americans. Unfortunately, many patients still struggle to find an option that effectively treats their attacks and is both safe and well tolerated, which creates a great need for new migraine medicines. Symbravo’s approval by the FDA provides a new medicine for physicians and patients that was designed to target key unmet needs in the migraine treatment space. The clinical data supporting its approval validates the additive benefit of Symbravo’s multi-mechanistic design and demonstrates its potential to make a meaningful difference for the migraine community.”
Susan Doughty, Executive Director of the Coalition for Headache and Migraine Patients (CHAMP), added, “Migraine is one of the most misunderstood and stigmatized neurological diseases, despite the fact that one in four households in the U.S. includes someone living with it. This widespread lack of understanding creates unnecessary barriers for individuals seeking proper diagnosis, care, and treatment. CHAMP, alongside our 20 plus dedicated coalition organizations and patient advocates, is committed to empowering the migraine community by providing education, reducing stigma, and advocating for fair and equitable access to treatment options. The approval of Symbravo as a new acute treatment for migraine is an important step forward, offering a new option for people seeking relief. We also see this moment as an opportunity to continue to shine a bright light on migraine, fostering greater awareness and helping to dismantle the stigma that so often surrounds this disease.”
The FDA approval of Symbravo is based on the results of the Phase 3 MOMENTUM trial that treated migraine of moderate and severe pain intensity, the Phase 3 INTERCEPT trial that treated migraine when the initial pain was mild, and the Phase 3 MOVEMENT long-term open label safety trial. In this comprehensive clinical program, over 21,000 migraine attacks were treated with Symbravo.
In the MOMENTUM trial, Symbravo demonstrated a statistically significantly greater percentage of patients achieving pain freedom and freedom from their most bothersome symptom (photophobia, phonophobia, nausea) 2 hours after dosing compared to placebo. Symbravo also demonstrated statistical superiority for pain relief (reduction of moderate or severe pain to no pain or mild pain) and the ability to perform normal daily activities. The benefits of pain freedom at 2 hours were sustained through 24 and 48 hours for many patients. In a head-to-head comparison, Symbravo demonstrated statistically significant superiority compared to rizatriptan on sustained pain freedom from 2 to 24 hours. Notably, these benefits were seen with only a single dose of Symbravo. In the MOMENTUM trial, 77% of patients treated with Symbravo did not require rescue medication within 24 hours post dose.
In the INTERCEPT trial, Symbravo demonstrated a statistically significantly greater percentage of patients achieving pain freedom and freedom from their most bothersome symptom (photophobia, phonophobia, nausea) 2 hours after dosing compared to placebo. The benefits of pain freedom at 2 hours were sustained through 24 and 48 hours for many patients. Notably, these benefits were seen with only a single dose of Symbravo. In the INTERCEPT trial, 85% of patients treated with Symbravo did not require rescue medication within 24 hours post dose.
The most common adverse reactions (≥1% and greater than placebo) in the controlled studies were somnolence and dizziness, being reported each in 2% and 1% of patients in the Symbravo and placebo arms, respectively. The long-term safety of Symbravo was demonstrated in the MOVEMENT trial, which assessed 706 patients dosing intermittently for up to 12 months and treating at least 2 migraines per month with Symbravo.
Symbravo is engineered with Axsome’s patented MoSEICTM (Molecular Solubility Enhanced Inclusion Complex) rapid absorption technology. MoSEIC results in a five times faster median time to maximum plasma concentration for meloxicam while maintaining a long plasma half-life, enabling meloxicam’s use as a new molecular entity for the acute treatment of migraine. Symbravo is protected by a robust patent estate extending out to at least 2040.