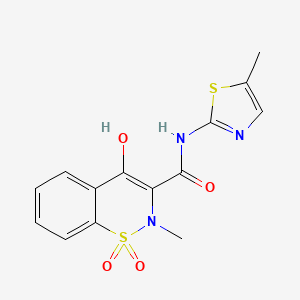
IntelGenx Corp. (TSX:IGX)(OTCQB:IGXT), announced the U.S. Food and Drug Administration approval of RizaFilm® VersaFilm® 505(b)(2) new drug application (NDA) for the treatment of acute migraine.
RizaFilm® is a proprietary oral thin film formulation of rizatriptan benzoate, the active ingredient in Merck & Co.'s Maxalt®. The global migraine drugs market was valued at nearly $3 billion in 2021 and is expected to reach nearly $11 billion by 2030, representing a compound annual growth rate of 15.6%.
In December 2018, IntelGenx entered into a definitive licensing, development and supply agreement with Gensco® Pharma (“Gensco”) for the exclusive commercialization of RizaFilm® in the United States. Under the terms of the agreement, IntelGenx is entitled to receive royalty payments based on net profits of RizaFilm®; and is eligible to receive pre-specified payments upon the achievement of certain regulatory and commercial milestones.
“Following a successful pre-approval inspection by the FDA of our Montreal manufacturing facility earlier this month, we are thrilled to reach this milestone and excited to soon introduce what will be the first oral thin film for the treatment of acute migraines available in the U.S.,” said Andre Godin, IntelGenx’s President and CFO. “According to the American Migraine Foundation, 39 million or 12% of Americans suffer from migraine, which is the second leading cause of disability nationwide. We are looking forward to working with our commercialization partner, Gensco, to bring this innovative migraine therapeutic to patients seeking convenient administration and quick relief from their pain. In addition to these benefits, RizaFilm® is well suited to the approximately 80% of patients who have migraine-related nausea3, as well as those who have difficulty swallowing.”