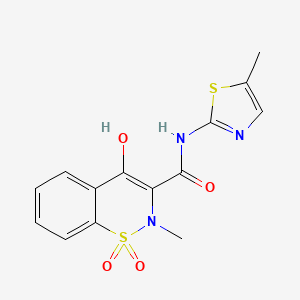
Pfizer Inc. (NYSE: PFE) announced the U.S. Food and Drug Administration (FDA) approval of Zavzpret (zavegepant), the first and only calcitonin gene-related peptide (CGRP) receptor antagonist nasal spray for the acute treatment of migraine with or without aura in adults. In its pivotal Phase 3 study, Zavzpret was statistically superior to placebo on the co-primary endpoints of pain freedom and freedom from most bothersome symptom at two hours post-dose. The pivotal study also demonstrated pain relief as early as 15 minutes in a prespecified secondary endpoint versus placebo.
“The FDA approval of Zavzpret marks a significant breakthrough for people with migraine who need freedom from pain and prefer alternative options to oral medications,” said Angela Hwang, Chief Commercial Officer, President, Global Biopharmaceuticals Business, Pfizer. “Zavzpret underscores Pfizer’s commitment to delivering an additional treatment option to help people with migraine gain relief and get back to their daily lives. Pfizer will continue to build its migraine franchise to further support the billions of people worldwide impacted by this debilitating disease.”
The FDA approval is based on two pivotal randomized, double-blind, placebo-controlled studies that established the efficacy, tolerability and safety profiles of Zavzpret for the acute treatment of migraine. In these studies, Zavzpret was statistically superior to placebo on the co-primary endpoints of pain freedom (defined as a reduction of moderate or severe headache pain to no headache pain) and freedom from most bothersome symptom at two hours post-dose (defined as the absence of the self-identified most bothersome symptom). The pivotal Phase 3 study published in The Lancet Neurology found Zavzpret showed broad efficacy by also demonstrating statistically significant superiority to placebo across 13 of 17 prespecified secondary outcome measures, including early time point endpoints (e.g., 15 and 30-minute pain relief and return to normal function at 30 minutes), return to normal function at 2 hours, and durable efficacy endpoints (e.g., 2-24 and 2-48 hour sustained pain freedom and sustained pain relief). On the 14th endpoint, return to normal function at 15 minutes post-dose, the difference between Zavzpret and placebo was not significant. Consequently, in keeping with the trial’s statistical analysis plan, the remaining secondary endpoints were not formally tested.
“When a migraine hits, it has a significant negative impact on a person’s daily life,” said Kathleen Mullin, M.D., Associate Medical Director at New England Institute for Neurology & Headache. “Among my migraine patients, one of the most important attributes of an acute treatment option is how quickly it works. As a nasal spray with rapid drug absorption, Zavzpret offers an alternative treatment option for people who need pain relief or cannot take oral medications due to nausea or vomiting, so they can get back to normal function quickly.”
Zavzpret was well tolerated in clinical trials. The most common adverse reactions reported in at least 2% of patients treated with Zavzpret and at a frequency greater than placebo were taste disorders (includes dysgeusia and ageusia), nausea, nasal discomfort and vomiting. Zavzpret is contraindicated in patients with a history of hypersensitivity to zavegepant or to any of its components. Hypersensitivity reactions, including facial swelling and urticaria, have occurred with Zavzpret in clinical studies.
https://en.wikipedia.org/wiki/Zavegepant#/media/File:Zavegepant.svg