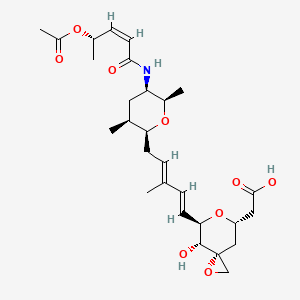
Thursday, July 21, 2022
FDA Approves Amvuttra (vutrisiran) for the Treatment of the Polyneuropathy of Hereditary Transthyretin-Mediated Amyloidosis in Adults
Friday, March 11, 2022
FDA Approves Leqvio (inclisiran), First-in-Class siRNA to Reduce Low-Density Lipoprotein Cholesterol (LDL-C)
"Leqvio is a revolutionary approach to lower LDL-C, and creates new possibilities for how healthcare systems can impact cardiovascular disease, a defining public health challenge of our time," said Vas Narasimhan, Novartis CEO. "We now have the opportunity, working together with partners, to provide this first-ever approved LDL-C–lowering siRNA-based therapy to tackle ASCVD at scale across the United States."
Leqvio is indicated in the United States as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with clinical atherosclerotic cardiovascular disease (ASCVD) or heterozygous familial hypercholesterolemia (HeFH) who require additional lowering of LDL-C. The effect of Leqvio on cardiovascular morbidity and mortality is being explored in clinical trials currently underway.
"ASCVD is a substantial public health burden affecting 30 million Americans," said Norman Lepor, MD, a Los Angeles based cardiologist and a clinical investigator in the Phase III clinical program for Leqvio. "As a first-of-its-kind siRNA therapy, Leqvio works differently than other cholesterol treatments, with twice-yearly dosing that makes it a compelling option for the millions of people with ASCVD already on cholesterol-lowering medications struggling to reach their LDL-C target."
Leqvio reduces the amount of LDL-C in the bloodstream by improving the liver's natural ability to prevent the production of a protein that plays a role in keeping circulating cholesterol levels high6,7. It is a subcutaneous injection given by a healthcare provider with an initial dose, then again at three months, and then every six months1. This approach may help those who have trouble sticking to medicines that are self-administered and have greater dosing frequency. Leqvio will be available in early January 2022.
"People with ASCVD have most likely experienced a heart attack or stroke from high cholesterol, causing a burden on the family and having a negative impact on lives," said Andrea Baer, Executive Director of The Mended Hearts, Inc. "One of the first steps to improving patients' health is to manage high cholesterol and we're encouraged that this new twice-a-year treatment offers a new option."
The FDA approval was based on results from the comprehensive Phase III ORION-9, -10 and -11 clinical trials, in which all 3,457 participants with ASCVD or HeFH had elevated LDL-C while receiving a maximally tolerated dose of statin therapy2,3. In the Phase III trials at month 17, Leqvio delivered effective and sustained LDL-C reduction of up to 52% vs. placebo and was reported to be well-tolerated with a safety profile shown to be comparable to placebo2,3. The most common side effects were mild to moderate injection site reaction (including pain, redness and rash), joint pain, urinary tract infection, diarrhea, chest cold, pain in legs or arms and shortness of breath2,3.
Novartis has obtained global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals, a leader in RNAi therapeutics.
Wednesday, January 26, 2022
FDA Approves Leqvio (inclisiran), First-in-Class siRNA to Reduce Low-Density Lipoprotein Cholesterol (LDL-C)
Novartis announced the US Food and Drug Administration (FDA) approval of Leqvio® (inclisiran), the first and only small interfering RNA (siRNA) therapy to lower low-density lipoprotein cholesterol (also known as bad cholesterol or LDL-C) with two doses a year, after an initial dose and one at three months.
"Leqvio is a revolutionary approach to lower LDL-C, and creates new possibilities for how healthcare systems can impact cardiovascular disease, a defining public health challenge of our time," said Vas Narasimhan, Novartis CEO. "We now have the opportunity, working together with partners, to provide this first-ever approved LDL-C–lowering siRNA-based therapy to tackle ASCVD at scale across the United States."
Leqvio is indicated in the United States as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with clinical atherosclerotic cardiovascular disease (ASCVD) or heterozygous familial hypercholesterolemia (HeFH) who require additional lowering of LDL-C. The effect of Leqvio on cardiovascular morbidity and mortality is being explored in clinical trials currently underway.
"ASCVD is a substantial public health burden affecting 30 million Americans," said Norman Lepor, MD, a Los Angeles based cardiologist and a clinical investigator in the Phase III clinical program for Leqvio. "As a first-of-its-kind siRNA therapy, Leqvio works differently than other cholesterol treatments, with twice-yearly dosing that makes it a compelling option for the millions of people with ASCVD already on cholesterol-lowering medications struggling to reach their LDL-C target."
Leqvio reduces the amount of LDL-C in the bloodstream by improving the liver's natural ability to prevent the production of a protein that plays a role in keeping circulating cholesterol levels high6,7. It is a subcutaneous injection given by a healthcare provider with an initial dose, then again at three months, and then every six months1. This approach may help those who have trouble sticking to medicines that are self-administered and have greater dosing frequency. Leqvio will be available in early January 2022.
"People with ASCVD have most likely experienced a heart attack or stroke from high cholesterol, causing a burden on the family and having a negative impact on lives," said Andrea Baer, Executive Director of The Mended Hearts, Inc. "One of the first steps to improving patients' health is to manage high cholesterol and we're encouraged that this new twice-a-year treatment offers a new option."
The FDA approval was based on results from the comprehensive Phase III ORION-9, -10 and -11 clinical trials, in which all 3,457 participants with ASCVD or HeFH had elevated LDL-C while receiving a maximally tolerated dose of statin therapy2,3. In the Phase III trials at month 17, Leqvio delivered effective and sustained LDL-C reduction of up to 52% vs. placebo and was reported to be well-tolerated with a safety profile shown to be comparable to placebo2,3. The most common side effects were mild to moderate injection site reaction (including pain, redness and rash), joint pain, urinary tract infection, diarrhea, chest cold, pain in legs or arms and shortness of breath2,3.
Novartis has obtained global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals, a leader in RNAi therapeutics.
Monday, March 1, 2021
FDA Approves Amondys 45 (casimersen) Injection for the Treatment of Duchenne Muscular Dystrophy (DMD) in Patients Amenable to Skipping Exon 45
In continuation of my update on antisense oligonucleotides
Sarepta Therapeutics, Inc. the leader in precision genetic medicine for rare diseases, today announced that the U.S. Food and Drug Administration (FDA) has approved Amondys 45 (casimersen). Amondys 45 is an antisense oligonucleotide from Sarepta’s phosphorodiamidate morpholino oligomer (PMO) platform, indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients with a confirmed mutation amenable to exon 45 skipping. This indication is based on a statistically significant increase in dystrophin production in skeletal muscle observed in patients treated with Amondys 45, which is reasonably likely to predict clinical benefit for those patients who are exon 45 amenable. Consistent with the accelerated approval pathway, the continued approval of Amondys 45 may be contingent on confirmation of a clinical benefit in confirmatory trials.
The ESSENCE trial – a placebo-controlled confirmatory trial to support the Amondys 45 approval – is ongoing and expected to conclude in 2024.
Although kidney toxicity was not observed in the clinical studies with Amondys 45, kidney toxicity, including potentially fatal glomerulonephritis, has been observed after administration of some antisense oligonucleotides. Kidney function should be monitored in patients taking Amondys 45. In the clinical trial, the most common adverse reactions observed in at least 20% of patients treated with Amondys 45 and at least 5% more frequently than in placebo were (Amondys 45, placebo): upper respiratory tract infections (65%, 55%), cough (33%, 26%), fever (33%, 23%), headache (32%, 19%), joint pain (21%, 10%), and pain in mouth and throat (21%, 7%).
“This is an important day for Sarepta and, far more importantly, for the patients that we serve. After years of scientific commitment, investment and development, the approval of Amondys 45, Sarepta’s third approved RNA therapy, offers treatment to the 8% of the DMD community who have a confirmed exon 45 amenable mutation,” said Doug Ingram, president and chief executive officer, Sarepta. “Along with our other approved RNA therapies, we can now offer treatment options for nearly 30% of Duchenne patients in the U.S. And our commitment to bring therapies to the greatest percentage of the DMD community as soon as possible continues.”
“Decades of research and commitment have fueled and now accelerate our progress towards new treatments for Duchenne,” said Marissa Penrod, founder of Team Joseph and parent of an 18-year old with Duchenne. “The extraordinary diligence and persistence of the Duchenne community – patients and families, clinicians and researchers – have led us to today’s approval, where we now have exon-skipping treatments for almost a third of those with Duchenne.”
Tuesday, March 24, 2020
FDA Approves Vyondys 53 (golodirsen) Injection for the Treatment of Duchenne Muscular Dystrophy (DMD) in Patients Amenable to Skipping Exon 53

“Today is monumental for Sarepta and, more importantly, for the DMD community,” said Doug Ingram, president and chief executive officer, Sarepta. “Vyondys 53, our second approved exon-skipping RNA therapy for DMD, may treat up to 8% of the DMD community, representing those patients who have a confirmed exon 53 amenable mutation. Along with EXONDYS 51® (eteplirsen), we now offer treatment options for approximately 20% of those with DMD in the U.S.”
“With the approval of Vyondys 53, up to another 8% of Duchenne families will have a therapy to treat this devastating disease,” said Pat Furlong, founding president and chief executive officer, Parent Project Muscular Dystrophy (PPMD). “For 25 years, PPMD has been working with researchers, clinicians, industry, and the Duchenne community to find treatments for all people living with Duchenne. And while we need to ensure that these approved therapies are accessible for patients, today we celebrate this approval and thank Sarepta for their continued leadership in the fight to end Duchenne.”
About Vyondys 53
Wednesday, March 4, 2020
FDA Approves Givlaari (givosiran) for Acute Hepatic Porphyria
“Adults with AHP now have a new treatment option that has demonstrated the ability to reduce the frequency of porphyria attacks by specifically addressing factors associated with attacks and other disease manifestations of AHP,” said Manisha Balwanii, M.D., M.S, Associate Professor of the Department of Genetics and Genomic Sciences and Department of Medicine at the Icahn School of Medicine at Mount Sinai and principal investigator of the ENVISION study. “With the approval of Givlaari, and based on the efficacy data from the ENVISION study, I hope to see my patients and those across the country be able to live more normal lives with fewer porphyria attacks.”
“The FDA approval of Givlaari is an important milestone for our community, as we now have a new treatment option for adults living with acute hepatic porphyria,” said Kristen Wheeden, Executive Director, American Porphyria Foundation. “AHP can have a profound impact on the lives of patients and their families. Porphyria attacks are associated with severe, incapacitating pain, often requiring hospitalization for management. In addition, many patients struggle on a daily basis with chronic symptoms related to their disease. The approval of Givlaari is exciting for our community.”
Saturday, May 18, 2019
FDA Approves Dovato (dolutegravir/lamivudine) for HIV-1 Infection

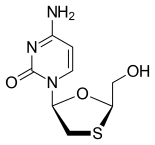
Pedro Cahn, principal investigator for the GEMINI study program said: “People are now living longer with HIV and will spend a lifetime taking drugs to suppress their virus. The approval of the fixed dose combination of dolutegravir and lamivudine, a complete, single-tablet, two-drug regimen, marks a pivotal moment in the treatment of HIV-1. Treatment-naïve people living with the virus have a powerful option that delivers non-inferior efficacy to a dolutegravir-based three-drug regimen, allowing them to take fewer ARVs and get and remain suppressed.”
About Dovato (dolutegravir/lamivudine)
https://en.wikipedia.org/wiki/Lamivudine
Wednesday, October 17, 2018
FDA Approves Onpattro (patisiran) Targeted RNA-based Therapy for Polyneuropathy Caused by hATTR

“This approval is part of a broader wave of advances that allow us to treat disease by actually targeting the root cause, enabling us to arrest or reverse a condition, rather than only being able to slow its progression or treat its symptoms. In this case, the effects of the disease cause a degeneration of the nerves, which can manifest in pain, weakness and loss of mobility,” said FDA Commissioner Scott Gottlieb, M.D. “New technologies like RNA inhibitors, that alter the genetic drivers of a disease, have the potential to transform medicine, so we can better confront and even cure debilitating illnesses. We’re committed to advancing scientific principles that enable the efficient development and review of safe, effective and groundbreaking treatments that have the potential to change patients’ lives.”
“There has been a long-standing need for a treatment for hereditary transthyretin-mediated amyloidosis polyneuropathy. This unique targeted therapy offers these patients an innovative treatment for their symptoms that directly affects the underlying basis of this disease,” said Billy Dunn, M.D., director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research.
FDA Approves Onpattro (patisiran) Targeted RNA-based Therapy for Polyneuropathy Caused by hATTR
Thursday, January 4, 2018
Garlic compound can combat robust bacteria in patients with chronic infections
'We really believe this method can lead to treatment of patients, who otherwise have poor prospects. Because chronic infections like cystic fibrosis can be very robust. But now we, together with a private company, have enough knowledge to further develop the garlic drug and test it on patients', says Assistant Professor Tim Holm Jakobsen from the Costerton Biofilm Center at the Department of Immunology and Microbiology.
'The two types of bacteria we have studied are very important. They are called Staphylococcus aureus and Pseudomonas aeruginosa. They actually belong to two very different bacteria families and are normally fought using different methods. But the garlic compound is able to fight both at once and therefore may prove an effective drug when used together with antibiotics', says Tim Holm Jakobsen.
Wednesday, November 22, 2017
FDA Approves Juluca, First Two-Drug Regimen for HIV Patients
 rilpivirine
rilpivirine  Dolutegravir
Dolutegravir“Limiting the number of drugs in any HIV treatment regimen can help reduce toxicity for patients,” said Debra Birnkrant, MD, director of the Division of Antiviral Products in the FDA’s Center for Drug Evaluation and Research.
Tuesday, September 5, 2017
FDA approves first drug to treat children and adults with spinal muscular atrophy
"There has been a long-standing need for a treatment for spinal muscular atrophy, the most common genetic cause of death in infants, and a disease that can affect people at any stage of life," said Billy Dunn, M.D., director of the Division of Neurology Products in the FDA's Center for Drug Evaluation and Research. "As shown by our suggestion to the sponsor to analyze the results of the study earlier than planned, the FDA is committed to assisting with the development and approval of safe and effective drugs for rare diseases and we worked hard to review this application quickly; we could not be more pleased to have the first approved treatment for this debilitating disease."
Friday, June 2, 2017
New chemical compound could potentially be used to treat Ebola virus infection
"We postulate that emergency administration of such an antiviral therapeutics during an outbreak would inhibit virus dissemination and spread in infected individuals, thus slowing disease progression and allowing the immune system more time to mount a robust response to effectively combat and clear the infection."
"This work is exciting to me since it may translate our basic science work into a potential product or therapeutic," concluded Dr. Harty.
Wednesday, February 1, 2017
Rice University researchers synthesize new anti-cancer agent