The FDA has approved the first complete treatment regimen containing only two drugs to treat certain adults with human immunodeficiency virus type 1 (HIV-1) instead of the three or more drugs included in standard HIV treatment.
Juluca (dolutegravir/rilpivirine, ViiV Healthcare) is a fixed-dose tablet approved to treat adults with HIV-1 infections whose virus is currently suppressed (HIV-1 RNA less than 50 copies per mL) on a stable regimen for at least six months, with no history of treatment failure and no known substitutions associated with resistance to the individual components of the new combination. Dolutegravir 50 mg (ViiV Healthcare) is an integrase strand transfer inhibitor, and rilpivirine 25 mg (Janssen Therapeutics) is a non-nucleoside reverse transcriptase inhibitor.
 rilpivirine
rilpivirine  Dolutegravir
Dolutegravir“Limiting the number of drugs in any HIV treatment regimen can help reduce toxicity for patients,” said Debra Birnkrant, MD, director of the Division of Antiviral Products in the FDA’s Center for Drug Evaluation and Research.
HIV weakens a person’s immune system by destroying important cells that fight disease and infection. According to the Centers for Disease Control and Prevention, an estimated 1.1 million people in the United States are living with HIV, and the disease remains a significant cause of death for certain populations.
This FDA approval is based primarily on data from two pivotal phase 3 clinical trials, SWORD-12 and SWORD-2,2 which showed the two-drug regimen achieved non-inferior viral suppression (HIV-1 RNA less than 50 copies per mL) at 48 weeks compared with patients’ three- or four-drug current antiretroviral regimen (CAR) in both pooled and individual analyses of the SWORD-1 and SWORD-2 studies (dolutegravir/rilpivirine 486/513 [95%], CAR 485/511 [95%]; adjusted difference, –0.2%; 95% confidence interval, –3.0% to 2.5%, pooled analysis). Virological suppression rates were similar between treatment arms. Drug related adverse events and adverse events leading to withdrawal occurred in low frequencies in both arms of the study, but more frequently in the investigational arm.
The most common side effects in patients taking Juluca were diarrhea and headache. Serious side effects include skin rash and allergic reactions, liver problems, and depression or mood changes. Juluca should not be given with other anti-HIV drugs and may have drug interactions with other commonly used medications.
Ref : https://www.viivhealthcare.com/media/press-releases/2017/november/viiv-healthcare-announces-us-fda-approval-for-juluca.aspx

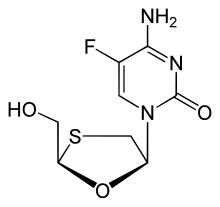 Emtricitabine
Emtricitabine 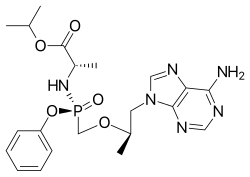 Tenofovir alafenamide
Tenofovir alafenamide