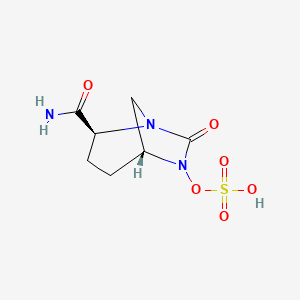
Nabriva Therapeutics plc (NASDAQ: NBRV), a biopharmaceutical company engaged in the commercialization and development of innovative anti-infective agents to treat serious infections, announced the U.S. Food and Drug Administration (FDA) approval of Nabriva’s new drug applications for the oral and intravenous (IV) formulations of Xenleta (lefamulin) for the treatment of community-acquired bacterial pneumonia (CABP) in adults. As the first IV and oral antibiotic with a novel mechanism of action approved by the FDA in nearly two decades, Xenleta represents an important new empiric monotherapy treatment option for adults with CABP.
“Today’s approval of Xenleta is a significant breakthrough in the collective fight against the growing threat of antimicrobial resistance and provides a desperately needed IV and oral empiric monotherapy treatment option for adults with CABP,” said Ted Schroeder, chief executive officer of Nabriva Therapeutics. “We are especially proud of this approval because Xenleta was discovered in our labs over a decade ago and the entire development program was designed and executed by our dedicated and passionate team. We are indebted to the patients and researchers who collaborated with us and are excited to bring to patients and healthcare providers a novel, short course, empiric monotherapy treatment option for CABP. Xenleta has a mechanism of action that is different than other approved antibiotics, resulting in a low propensity for the development of resistance, as well as a lack of cross-resistance with the beta-lactam, fluoroquinolone, glycopeptide, macrolide, and tetracycline antibiotic classes. Xenleta has a targeted in vitro spectrum of activity against the most common causative Gram-positive, Gram-negative and atypical pathogens associated with CABP, which aligns with the principles of antimicrobial stewardship.”
“The gravity of antimicrobial resistance cannot be overstated, particularly in the context of treating pneumonia,” said Julio Ramirez, MD, FACP, Professor of Medicine, and Chief within the Division of Infectious Diseases, University of Louisville School of Medicine. “As an infectious disease specialist who treats CABP patients in the hospital setting, I am grateful to have both a new IV and oral option that gives me confidence that my patients will continue to receive appropriate therapy once they are discharged from the hospital.”
Xenleta is available for oral (600 mg every 12 hours) and IV (150 mg every 12 hours) administration with a short 5-to-7 day course of therapy. Clinicians can initiate patients on IV or oral therapy, allowing for potential avoidance of hospitalization, or can transition from IV to oral therapy, which may expedite discharge from the hospital. Currently, the median length of stay for patients with pneumonia is 3-to-4 days, resulting in approximately $17 billion in hospital costs per year in the United States. The opportunity to avoid a hospital admission or to discharge a patient earlier on oral therapy benefits patients and may result in significant savings to the health system.
Both the IV and oral formulations of Xenleta were granted Qualified Infectious Disease Product (QIDP) and Fast Track designation by the FDA. The FDA approval was based on a clinical development program supported by a robust data package, including two pivotal, Phase 3 trials (known as LEAP 1 and LEAP 2) that evaluated the safety and efficacy of IV and oral Xenleta compared to moxifloxacin in the treatment of adults with CABP. LEAP 1 was designed to evaluate 5-to-7 days of IV/oral therapy of Xenleta versus 7-days of IV/oral moxifloxacin, with or without linezolid, with both treatment groups having the option to switch from IV to oral administration after 3-days. LEAP 2 evaluated 5-days of oral Xenleta versus 7-days of oral moxifloxacin. LEAP 1 showed comparable efficacy with moxifloxacin, with or without linezolid, while LEAP 2 showed comparable efficacy with moxifloxacin, with two fewer days of therapy. Xenleta was generally well tolerated in both LEAP 1 and LEAP 2.
“Emergency departments across the country treat hundreds of thousands of patients with CABP each year. Many of these patients, especially elderly patients with comorbidities, are admitted solely because of the lack of an effective and well-tolerated oral treatment option” said Philip Giordano, MD, Vice Chairman of Emergency Medicine at the Orlando Regional Medical Center. “With a new oral antibiotic option that has been shown to be as effective as a respiratory fluoroquinolone, possessing a favorable side effect profile, we can consider sending more patients home directly from the emergency department and avoid costly hospitalizations, which is good for both patient care and the health system.”
Pneumonia is an infection of the lung that can be serious and fatal, especially among older adult patients with comorbidities. There are approximately five million cases of pneumonia in the U.S. each year, and pneumonia is the fifth leading cause of hospitalization and one of the leading causes of infection-related death. Streptococcus pneumoniae is the most common cause of bacterial pneumonia in the U.S. According to recent data from the SENTRY Antimicrobial Surveillance Program, in the U.S., approximately 30 to 60 percent of S. pneumoniae, depending on region, are macrolide resistant. In addition to macrolides, fluoroquinolones are another common treatment option for CABP. This broad-spectrum class is an effective option, however fluoroquinolones carry boxed warnings for several significant safety concerns.
Nabriva expects Xenleta will be available through major U.S. specialty distributors in mid-September 2019. Xenleta will have a wholesale acquisition (WAC) price of $205 per IV patient treatment day and $275 per oral patient treatment day.
“Offering clinicians and patients a new treatment option for CABP that addresses the urgent and growing threat of antimicrobial resistance is our top priority. With that in mind, our team has been working hard to make Xenleta available in the weeks ahead to ensure that patients with CABP who can benefit from this new treatment option can access it,” said Schroeder.
About Xenleta
Xenleta (lefamulin) is a first-in-class semi-synthetic pleuromutilin antibiotic for systemic administration in humans discovered and developed by the Nabriva Therapeutics team. It is designed to inhibit the synthesis of bacterial protein, which is required for bacteria to grow. Xenleta’s binding occurs with high affinity, high specificity and at molecular sites that are different than other antibiotic classes. Based on results from its two global, Phase 3 clinical trials, Nabriva Therapeutics believes Xenleta is well-positioned for use as a first-line monotherapy for the treatment of CABP due to its novel mechanism of action, targeted spectrum of activity, resistance profile, achievement of substantial drug concentration in lung tissue and fluid, availability of oral and IV formulations and a generally well-tolerated safety profile. Nabriva Therapeutics believes XENLETA represents a potentially important new treatment option for the approximately five million adults in the United States diagnosed with pneumonia each year.
In LEAP 1 and LEAP 2 (pooled), the median age of patients treated with Xenleta was 61 (range 19-97) years; 42% of patients were 65 years or older and 18% of patients were 75 years or older. In both trials, approximately half of Xenleta-treated patients had impaired renal function and the most common other comorbidities included hypertension, asthma/COPD and diabetes mellitus. These baseline characteristics were broadly representative of the adult patient population with CABP.
In LEAP 1, Xenleta demonstrated non-inferiority compared to moxifloxacin, with or without linezolid, for the FDA primary endpoint of early clinical response (ECR) assessed 72 to 120 hours following initiation of therapy in the intent to treat (ITT) patient population (ECR rate = 87.3% for Xenleta and 90.2% for moxifloxacin, with or without linezolid; treatment difference -2.9 [95% confidence interval (CI) -8.5, 2.8]). In LEAP 2, 5-days of oral Xenleta also demonstrated non-inferiority to 7-days of oral moxifloxacin for the ECR endpoint in the ITT population (ECR rate = 90.8% for Xenleta and 90.8% moxifloxacin; treatment difference 0.1 [95% confidence interval (CI) -4.4, 4.5]). Importantly, high-risk patients 65 years and older achieved a similar ECR rate as those less than 65 years of age. The most common adverse reactions in patients receiving Xenleta in LEAP 1 (IV and oral) were administration site reactions, hepatic enzyme elevation, nausea, hypokalemia, insomnia, and headache in LEAP 1, and in LEAP 2 (oral only) diarrhea, nausea, vomiting and hepatic enzyme elevation. Few discontinuations due to adverse reactions were reported (3.3% in both treatment arms) and the 28-day mortality was low and balanced between treatment groups [8 patients (1.2%)] and [7 patients (1.1%)] for Xenleta and comparator, respectively from pooled data for LEAP 1 and LEAP 2.
https://www.drugbank.ca/drugs/DB12825
https://en.wikipedia.org/wiki/Lefamulin