Janssen Pharmaceuticals, Inc. (Janssen), announced the U.S. Food and Drug Administration (FDA) has approved Invokamet, a fixed-dose combination therapy of INVOKANA® (canagliflozin) and metformin hydrochloride, for first-line treatment of adults with type 2 diabetes. With this new approval, Invokamet may now be prescribed in adults with type 2 diabetes who are not already being treated with canagliflozin or metformin and may benefit from dual therapy.
 metformin
metformin 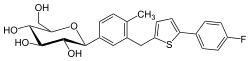 canagliflozin
canagliflozin
Invokamet, the first combination of a sodium glucose co–transporter 2 (SGLT2) inhibitor and metformin available in the United States, was previously approved by the FDA in August 2014 as an adjunct to diet and exercise to improve blood glucose control in adults with type 2 diabetes not adequately controlled by either canagliflozin or metformin, or who are already being treated with both medications separately.
“Physicians increasingly try to achieve greater initial blood sugar control by using dual therapy at the outset, versus single-agent therapy alone, especially for patients with higher A1C levels,” said John Anderson, M.D.*, Frist Clinic, Nashville, Tenn. “Invokamet combines two effective, complementary medicines—canagliflozin and metformin—into one convenient pill, to lower A1C significantly more than metformin alone.”
A1C is a measure of average blood glucose over the past two to three months; the American Diabetes Association recommends most adults with type 2 diabetes maintain A1C levels of 7 percent or less.[2]
The new Invokamet indication aligns with recent type 2 diabetes treatment guidelines, which recommend dual therapy for patients with higher A1C levels. Specifically, guidelines recommend dual therapy for patients who have an initial A1C level of 7.5 percent or higher;[3] and for those who have an initial level below 7.5 percent and do not achieve an A1C treatment goal after about three months on single therapy, often metformin.3,[4] In addition, dual or triple therapy is recommended as first-line therapy in asymptomatic patients with an initial A1C level above 9 percent.3
Studies have demonstrated that administration of Invokamet results in the same levels and effects of canagliflozin and metformin in the body as co-administration of corresponding doses of both drugs as individual tablets. Canagliflozin works with the kidneys to help adults with type 2 diabetes lose some sugar through the process of urination, and metformin decreases the production of glucose in the liver and improves the body's response to insulin. Invokamet should not be used in patients with type 1 diabetes or for the treatment of diabetic ketoacidosis.1
Invokamet is available in four dose strengths, in tablets containing canagliflozin 50 milligrams (mg) or 150 mg, and metformin 500 mg or 1000 mg. The recommended dosing is twice daily. The prescribing information for Invokamet also contains a boxed warning for lactic acidosis, a rare, but serious complication that can occur due to metformin accumulation.1
“The available doses of Invokamet allow physicians to tailor therapy for individual patient needs and offer an alternative for people living with type 2 diabetes who may be able to reduce the number of pills they take each day,” said Paul Burton, M.D., Ph.D., Vice President, Medical Affairs, Janssen. “This expansion marks an important milestone as we continue to study Invokamet and INVOKANA®—the number-one prescribed SGLT2 inhibitor with more than 8 million prescriptions to date—for the treatment of type 2 diabetes.”
Phase 3 Study Supports Expanded Indication
The expanded indication for Invokamet was based largely on a 26-week, double-blind, active-controlled, multicenter Phase 3 study in 1,186 adults with type 2 diabetes inadequately controlled with diet and exercise, and who had not been treated previously with any glucose-lowering medications. The participants were assigned randomly to one of five treatment groups: metformin hydrochloride extended release (MET), canagliflozin 100 mg (CANA100), canagliflozin 300 mg (CANA300), canagliflozin 100 mg + MET (CANA100/MET), or canagliflozin 300 mg + MET (CANA300/MET). The mean baseline A1C across all groups was 8.8 percent. The primary endpoint was the change in A1C. A report on the study findings was published in Diabetes Care in March 2016.[5]
After 26 weeks, participants in the CANA100/MET and CANA300/MET groups had significantly greater decreases in A1C compared to those in the CANA100, CANA300 and MET groups: 1.77 percent and 1.78 percent vs. 1.37 percent, 1.42 percent and 1.3 percent, respectively (p-values for all differences between the combination therapies vs. individual therapies less than 0.001). Additionally, significantly more participants in the CANA100/MET and CANA300/MET groups compared to the MET group achieved the goal of reducing A1C to less than 7 percent: 47 percent and 51 percent vs. 38 percent, respectively (p less than 0.05 for both combination groups vs. MET).1
Other Phase 3 Studies of Canagliflozin-Metformin Therapy
The co-administration of canagliflozin—INVOKANA®—and metformin has been evaluated in six other Phase 3 clinical studies that enrolled 4,732 patients with type 2 diabetes and who were already taking glucose-lowering medications. The studies showed that the combination of INVOKANA® and metformin lowered blood sugar and, in pre-specified secondary endpoints, was associated with significant reductions in body weight and systolic blood pressure.
In two studies comparing INVOKANA® plus metformin to current standard treatments plus metformin—one studying sitagliptin and the other studying glimepiride—INVOKANA® dosed at 300 mg provided greater reductions in A1C levels and body weight than either comparator. The overall incidence of adverse events was similar with INVOKANA® and the comparators.
Results from the Phase 3 studies showed that INVOKANA® was generally well tolerated, and the most common adverse events include genital yeast infections, urinary tract infections, and changes in urination. The most common adverse reactions due to initiation of metformin, as noted in the prescribing information for that medication, are diarrhea, nausea, vomiting, flatulence, asthenia, indigestion, abdominal discomfort, and headache. Hypoglycemia does not occur in patients receiving metformin alone under usual circumstances of use. INVOKANA® can increase the risk of hypoglycemia when combined with insulin or a medication that increases insulin levels (e.g., a sulfonylurea). Therefore, a lower dose of insulin or insulin-raising medication may be required to minimize the risk of hypoglycemia when used in combination with Invokamet.
About Type 2 Diabetes
Of the approximately 29 million people who have diabetes in the United States, 90 to 95 percent of them have type 2 diabetes, which is chronic and affects the body's ability to metabolize sugar (glucose), and is characterized by the inability of pancreatic beta cell function to keep up with the body's demand for insulin







 Saxagliptin and
Saxagliptin and