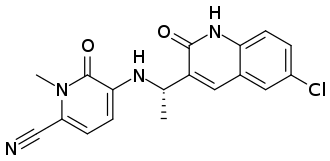
GSK plc (LSE/NYSE: GSK) announced the US Food and Drug Administration (FDA) approval of Jesduvroq (daprodustat), an oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI), for the once-a-day treatment of anemia due to chronic kidney disease (CKD) in adults who have been receiving dialysis for at least four months. Jesduvroq is the first innovative medicine for anemia treatment in over 30 years and the only HIF-PHI approved in the US, providing a new oral, convenient option for patients in the US with anemia of CKD on dialysis.
The FDA approval is based on results from the ASCEND-D trial, assessing the efficacy and safety of Jesduvroq for the treatment of anemia of CKD in patients on dialysis. Results were published in the New England Journal of Medicine with additional results published in the New England Journal of Medicine supplementary appendix.
The Safety Information for Jesduvroq includes a boxed warning for increased risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access. Jesduvroq increases the risk of thrombotic vascular events, including major adverse cardiovascular events (MACE). Targeting a hemoglobin level greater than 11 g/dL is expected to further increase the risk of death and arterial venous thrombotic events, as occurs with erythropoietin stimulating agents (ESAs), which also increase erythropoietin levels. No trial has identified a hemoglobin target level, dose of Jesduvroq, or dosing strategy that does not increase these risks. Use the lowest dose of Jesduvroq sufficient to reduce the need for red blood cell transfusions. Jesduvroq has not been shown to improve quality of life, fatigue, or patient well-being. Jesduvroq is not indicated for use as a substitute for red blood cell transfusions in patients who require immediate correction of anemia or for treatment of anemia of chronic kidney disease in patients who are not on dialysis.
CKD is an increasing global health burden affecting 700 million patients worldwide, with an estimated one in seven patients also developing anemia.1,2 When left untreated or undertreated, anemia of CKD is associated with poor clinical outcomes and leads to a substantial burden on patients and healthcare systems.3 There is an unmet need for oral treatment options with efficacy and safety comparable to current treatments.
LaVarne Burton, President and Chief Executive Officer, American Kidney Fund, said: “Anemia of CKD can be a debilitating condition that is challenging to manage. This news means that patients on dialysis who are living with anemia of CKD now have another treatment option to help manage their anemia.”
A marketing authorisation application for daprodustat is currently under review with the European Medicines Agency, with a regulatory decision anticipated in the first half of 2023. In June 2020, daprodustat tablets were approved by Japan’s Ministry of Health, Labour and Welfare for the treatment of patients with anemia of CKD. In Japan, the brand name for daprodustat is Duvroq, where it is the market leader and preferred HIF-PHI.
Tony Wood, President and Chief Scientific Officer, GSK, said: “Over the last several decades, there has been little innovation in anemia of CKD. We are proud to have developed Jesduvroq as a new oral treatment where there is a patient desire for more options.”
The FDA approval is based on results from the ASCEND-D trial, assessing the efficacy and safety of Jesduvroq for the treatment of anemia of CKD in patients on dialysis. Results were published in the New England Journal of Medicine with additional results published in the New England Journal of Medicine supplementary appendix.
The Safety Information for Jesduvroq includes a boxed warning for increased risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access. Jesduvroq increases the risk of thrombotic vascular events, including major adverse cardiovascular events (MACE). Targeting a hemoglobin level greater than 11 g/dL is expected to further increase the risk of death and arterial venous thrombotic events, as occurs with erythropoietin stimulating agents (ESAs), which also increase erythropoietin levels. No trial has identified a hemoglobin target level, dose of Jesduvroq, or dosing strategy that does not increase these risks. Use the lowest dose of Jesduvroq sufficient to reduce the need for red blood cell transfusions. Jesduvroq has not been shown to improve quality of life, fatigue, or patient well-being. Jesduvroq is not indicated for use as a substitute for red blood cell transfusions in patients who require immediate correction of anemia or for treatment of anemia of chronic kidney disease in patients who are not on dialysis.
CKD is an increasing global health burden affecting 700 million patients worldwide, with an estimated one in seven patients also developing anemia.1,2 When left untreated or undertreated, anemia of CKD is associated with poor clinical outcomes and leads to a substantial burden on patients and healthcare systems.3 There is an unmet need for oral treatment options with efficacy and safety comparable to current treatments.
LaVarne Burton, President and Chief Executive Officer, American Kidney Fund, said: “Anemia of CKD can be a debilitating condition that is challenging to manage. This news means that patients on dialysis who are living with anemia of CKD now have another treatment option to help manage their anemia.”
A marketing authorisation application for daprodustat is currently under review with the European Medicines Agency, with a regulatory decision anticipated in the first half of 2023. In June 2020, daprodustat tablets were approved by Japan’s Ministry of Health, Labour and Welfare for the treatment of patients with anemia of CKD. In Japan, the brand name for daprodustat is Duvroq, where it is the market leader and preferred HIF-PHI.
The FDA approval is based on results from the ASCEND-D trial, assessing the efficacy and safety of Jesduvroq for the treatment of anemia of CKD in patients on dialysis. Results were published in the New England Journal of Medicine with additional results published in the New England Journal of Medicine supplementary appendix.
The Safety Information for Jesduvroq includes a boxed warning for increased risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access. Jesduvroq increases the risk of thrombotic vascular events, including major adverse cardiovascular events (MACE). Targeting a hemoglobin level greater than 11 g/dL is expected to further increase the risk of death and arterial venous thrombotic events, as occurs with erythropoietin stimulating agents (ESAs), which also increase erythropoietin levels. No trial has identified a hemoglobin target level, dose of Jesduvroq, or dosing strategy that does not increase these risks. Use the lowest dose of Jesduvroq sufficient to reduce the need for red blood cell transfusions. Jesduvroq has not been shown to improve quality of life, fatigue, or patient well-being. Jesduvroq is not indicated for use as a substitute for red blood cell transfusions in patients who require immediate correction of anemia or for treatment of anemia of chronic kidney disease in patients who are not on dialysis.
CKD is an increasing global health burden affecting 700 million patients worldwide, with an estimated one in seven patients also developing anemia.1,2 When left untreated or undertreated, anemia of CKD is associated with poor clinical outcomes and leads to a substantial burden on patients and healthcare systems.3 There is an unmet need for oral treatment options with efficacy and safety comparable to current treatments.
LaVarne Burton, President and Chief Executive Officer, American Kidney Fund, said: “Anemia of CKD can be a debilitating condition that is challenging to manage. This news means that patients on dialysis who are living with anemia of CKD now have another treatment option to help manage their anemia.”
A marketing authorisation application for daprodustat is currently under review with the European Medicines Agency, with a regulatory decision anticipated in the first half of 2023. In June 2020, daprodustat tablets were approved by Japan’s Ministry of Health, Labour and Welfare for the treatment of patients with anemia of CKD. In Japan, the brand name for daprodustat is Duvroq, where it is the market leader and preferred HIF-PHI.
https://en.wikipedia.org/wiki/Daprodustat
https://go.drugbank.com/drugs/DB11682