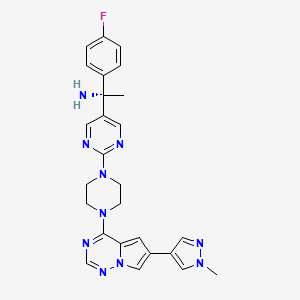
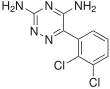
In continuation of my update on Ubrelvy (ubrogepant)
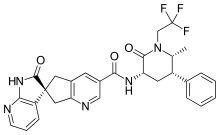
Allergan plc (NYSE: AGN), announced that the U.S. Food and Drug Administration (FDA) has approved a New Drug Application (NDA) for Ubrelvy (ubrogepant) for the acute treatment of migraine with or without aura in adults. Ubrelvy™ is the first and only orally-administered calcitonin gene-related peptide (CGRP) receptor antagonist (gepant) for the treatment of migraine attacks once they start. Migraine is a neurological disease characterized by intermittent migraine attacks with symptoms that are often incapacitating. Migraine afflicts 31 million Americans and is the third most common disease and second leading cause of disability worldwide.
"The FDA's approval of Ubrelvy™, a new oral option that is effective in the acute treatment of migraine attacks, is a much-welcomed development for me and for many who care for patients. I can offer my migraine patients a new treatment option that may work for them," said Dr. Peter Goadsby, Neurologist and Professor at King's College, London, and University of California, San Francisco, and a paid consultant for Allergan. "Perhaps even better, I am able to offer a new sense of hope for my patients who struggle for relief from this highly disabling problem."
In clinical trials supporting the FDA's approval, Ubrelvy™ provided quick pain relief for the majority of migraine patients. Ubrelvy™ also met co-primary endpoints of freedom from pain and freedom from the most bothersome symptom (nausea, hypersensitivity to light, or hypersensitivity to sound), a recent, more stringent standard of efficacy the FDA set in 2018. Ubrelvy™ provided lasting relief up to 24 hours as well. Ubrelvy™ works in a new way by blocking CGRP, a protein that is released during a migraine attack, from binding to its receptors. It works without constricting blood vessels, which some older treatments are known to do. Ubrelvy™ is non-narcotic, not scheduled, and does not have addiction potential. It has been approved with two dose strengths, 50 mg and 100 mg, and is specially designed so healthcare providers can provide a personalized treatment approach for appropriate patients.
"As someone living with migraine for 14 years, my life seems to be on pause when I experience a migraine attack," said Kristin Molacek, Ubrelvy™ clinical trial patient. "During the clinical trial, my experience with Ubrelvy™ was positive. It relieved the migraine symptoms that bothered me the most without serious side effects. We have needed this type of on-demand oral relief for a very long time, and I look forward to having the ability to better manage my migraine attacks."
"We are extremely pleased that Ubrelvy™ is now approved by the FDA. As the first oral gepant, Ubrelvy™ offers a new and different type of acute treatment option for people living with the debilitating pain and other symptoms of migraine," said David Nicholson, EVP and Chief R&D Officer, Allergan. "Its oral administration with two dose strengths allows for treatment flexibility and relief when a migraine attack occurs. As we continue to drive innovation in migraine treatment, we are very proud to offer patients another option, and we are confident that it will make a difference for those in need. At Allergan, we believe that migraine patients deserve access to all new medications for this debilitating disease."
About Ubrelvy (ubrogepant)
Ubrelvy (ubrogepant) is a novel, highly potent, orally-administered calcitonin gene-related peptide (CGRP) receptor antagonist (gepant) for the acute treatment of migraine with or without aura in adults that is an option for a wide range of patients who experience migraine attacks. It works in a new way by blocking CGRP, a protein released during a migraine attack, from binding to its receptors. It works without constricting blood vessels, which some older treatments are known to do. CGRP receptor antagonism is a completely new mechanism of action for the acute treatment of migraine.
The FDA approval for Ubrelvy is based on four clinical studies (ACHIEVE I, ACHIEVE II, UBR-MD-04 and 3110-105-002), which demonstrated efficacy, safety and tolerability of orally-administered Ubrelvy in the acute treatment of migraine. The two pivotal Phase 3 clinical trials (ACHIEVE I and ACHIEVE II) established the safety and efficacy profile of Ubrelvy. Both 50 mg and 100 mg dose strengths demonstrated significantly greater rates of pain freedom and freedom from the most bothersome migraine-associated symptom at two hours, compared with placebo.
Nausea was the most common adverse event reported in 1.7-4.1% of patients at various doses during the pivotal studies, compared to 1.6-2.0% of patients who received placebo. There were no serious adverse events within 48 hours of a dose. Additionally, the safety study (UBR-MD-04) reinforced the long-term safety and tolerability of Ubrelvy for both the 50 mg and 100 mg dose strengths. Our research shows that Ubrelvy was well tolerated with an adverse event profile similar to placebo.
https://en.wikipedia.org/wiki/Ubrogepant