A 59-year-old heart patient with dangerously high levels of cholesterol that could not be adequately reduced by statin drugs now has near-normal cholesterol levels, thanks to a new class of drugs that grew out of work done by UT Southwestern Medical Center researchers.
Two of these drugs, in a category known as PCSK9 inhibitors, were approved by the Food and Drug Administration last summer for use by some individuals with extremely high cholesterol levels.
"If you take the core patients who are at highest risk, it makes you appreciate how important this drug class is," said Dr. Amit Khera, Director of the Preventive Cardiology Program and Associate Professor of Internal Medicine at UT Southwestern.
Frank Brown of Dallas, grandfather of six and the owner of Frank's Wrecker Service in Dallas, has familial hypercholesterolemia, an inherited condition that causes high levels of cholesterol, especially low-density lipoprotein (LDL) cholesterol or "bad cholesterol." High levels of LDL cholesterol are strongly associated with heart disease.
Mr. Brown, with a history of two heart attacks, had been aggressively treated with multiple drugs to reduce his cholesterol levels, but they remained stubbornly high.
"When I first met Mr. Brown, he had a strong family history of heart disease, he had a cholesterol level that was ridiculously high with an LDL of 384, and he was having chest pains," said Dr. Amit Khera, who is Mr. Brown's cardiologist.
Dr. Khera, who holds the Dallas Heart Ball Chair in Hypertension and Heart Disease at UT Southwestern, was treating Mr. Brown with three cholesterol-lowering medications: a statin, which is a class of drugs that works by blocking a substance the body needs to make cholesterol; ezetimibe, a drug that blocks absorption of cholesterol in the intestine; and colesevelam, which sequesters bile acids. Even with this trio of medicines, Mr. Brown's LDL cholesterol level hovered around 200.

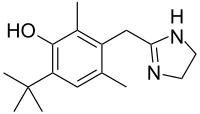
 (Prednisole)
(Prednisole) (Indomethacin)
(Indomethacin)
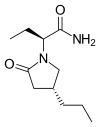
 (
( (Donepezil)
(Donepezil) 

 (Elbasvir)
(Elbasvir)  (Grazoprevir)
(Grazoprevir)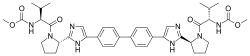
 Tetracaine
Tetracaine