In continuation of my update on daclatasvir
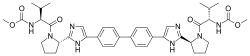
Bristol-Myers Squibb Company (NYSE:BMY) announced today that Daklinza (daclatasvir, 60 mg), an NS5A replication complex inhibitor, has been approved by the U.S. Food and Drug Administration (FDA) in combination with sofosbuvir (with or without ribavirin) in genotypes 1 and 3. The expanded label includes data in three additional challenging-to-treat patient populations: chronic hepatitis C virus (HCV) patients with HIV-1 coinfection, advanced cirrhosis, or post-liver transplant recurrence of HCV. The Daklinza plus sofosbuvir regimen is already available for the treatment of chronic HCV genotype 3, and is currently the only 12-week, once-daily all-oral treatment option for these patients. Sustained virologic response (SVR) rates are reduced in genotype 3 patients with cirrhosis receiving Daklinza and sofosbuvir for 12 weeks without ribavirin. The recommended dosage of Daklinza is 60 mg in combination with sofosbuvir with or without (+/-) ribavirin for 12 weeks.
