Chemists have designed a carbohydrate-based molecule that can surround and strangle bone cancer cells by self-assembling into a tangled web of nanofibers (J. Am. Chem. Soc. 2014, DOI: 10.1021/ ja5111893). The molecule spares healthy cells because its assembly is triggered by an enzyme that’s overexpressed on cancer cells.
The inspiration for spinning a molecular cage around cells came from nature, says Rein V. Ulijn of the City University of New York’s Hunter College. Many of the body’s cells are enmeshed in an extracellular matrix—a complex web of biomolecules that provides structure for tissues, facilitates intercellular communication, and traps nutrients. Scientists are developing molecules that spontaneously assemble into simpler versions of this matrix to provide a growth medium for cells, in particular for tissue engineering.
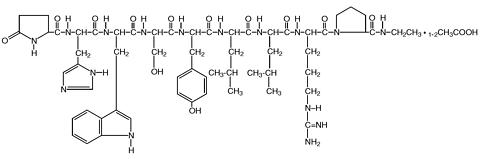
The field has focused mainly on self-assembling peptides. In a recent study, Bing Xu of Brandeis University and colleagues designed a nonnurturing peptide that aggregates and engulfs cancer cells only when its phosphate group is removed (Angew. Chem. Int. Ed. 2014, DOI: 10.1002/anie.201402216). The phosphate-free peptides have a hydrophilic end and a hydrophobic one, which allow them to assemble like lipids in a cell membrane. The negative charge on the phosphate groups creates electrostatic repulsion between the molecules and prevents this. This phosphate on-off switch is great for targeting cancer because some types of cancer cells overexpress alkaline phosphatase, an enzyme that cleaves phosphates.
Ref : http://onlinelibrary.wiley.com/doi/10.1002/anie.201402216/abstract