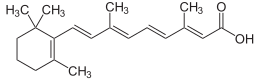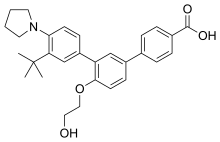
Galderma, a global leader focused on meeting the world's increasing skin health needs, announced today that the U.S. Food and Drug Administration (FDA) approved Aklief (trifarotene) Cream, 0.005% for the topical treatment of acne. Aklief Cream is the only topical retinoid that selectively targets retinoic acid receptor (RAR) gamma, the most common RAR found in the skin.1,2,3 Trifarotene is the first new retinoid molecule to receive U.S. FDA approval for the treatment of acne in more than 20 years.4,5,6 Aklief Cream is the first topical treatment specifically studied and proven to treat both facial (forehead, cheeks, nose and chin) and truncal (chest, shoulders and back) acne, offering healthcare professionals and acne patients another treatment option.
"While retinoids are foundational therapies to treat acne, there has been little innovation in decades.” said Sandra Johnson, M.D., FAAD, an investigator in the clinical trials of Aklief Cream and a dermatologist at Johnson Dermatology in Fort Smith, Arkansas. “With the approval of Aklief Cream, I am excited to offer my patients a unique, highly targeted retinoid that reduces inflammatory lesions on the face, back, chest and shoulders, that has also been shown to be safe and well-tolerated."
The FDA approval of Aklief Cream is supported by data from the two pivotal Phase 3 clinical trials of once-daily Aklief Cream in patients with moderate acne on the face and trunk.1 The two identical 12-week, randomized, multicenter, parallel group, double-blind, vehicle-controlled clinical trials of 2,420 patients showed that Aklief Cream significantly reduced inflammatory lesions as early as two weeks on the face and four weeks on the back, shoulders and chest compared to vehicle (p<0.05).1 Aklief Cream was well tolerated when used on the face, back, shoulders and chest.1 The most common adverse reactions (incidence >1%) included application site irritation, application site pruritus (itching) and sunburn.1
“The approval of Aklief Cream underscores our ability to bring new active molecules to the community, and innovate even in well-established therapeutic classes. It is consistent with our intent to change the paradigm of how even the most common and frustrating skin diseases are treated, including acne,” said Thibaud Portal PhD, Galderma Global Vice President, Prescription. “We are pleased to add this new treatment option, with proven efficacy in facial and truncal acne, to our innovative and differentiated portfolio of acne treatments.”
Aklief Cream is expected to be available in the United States in November 2019. It will be provided in a 45 gram pump. Galderma is working closely with payers, providers and pharmacy benefit managers to ensure broad and rapid access to Aklief Cream. The company will also offer a patient savings card program, Galderma CareConnect*.
*Galderma CareConnect is only available for commercially insured or uninsured patients. Patients who are enrolled in a government-run or government-sponsored healthcare plan with a pharmacy benefit are not eligible to use the Galderma CareConnect Patient Savings Card.