
In continuation of my update on Artesunate

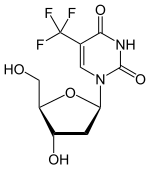 (Trifluridine)
(Trifluridine) 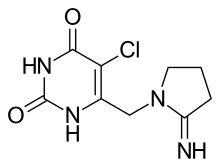 (Tipiracil)
(Tipiracil) Led by Randall Holcombe, MD, and Sofya Pintova, MD, both from Mount Sinai, the research team treated colon cancer cell lines with genistein and found that it inhibited cell growth and blocked Wnt signaling hyperactivity. The findings are counter to some other tumor types, such as breast, for which soy, because it has estrogen-like properties, increases the risk of developing tumors. Drs. Holcombe and Pintova are launching a clinical trial later this year for patients with metastatic colorectal cancer, which utilizes genistein in combination with chemotherapy based on this research.
Led by Randall Holcombe, MD, and Sofya Pintova, MD, both from Mount Sinai, the research team treated colon cancer cell lines with genistein and found that it inhibited cell growth and blocked Wnt signaling hyperactivity. The findings are counter to some other tumor types, such as breast, for which soy, because it has estrogen-like properties, increases the risk of developing tumors. Drs. Holcombe and Pintova are launching a clinical trial later this year for patients with metastatic colorectal cancer, which utilizes genistein in combination with chemotherapy based on this research.
"Genistein is a natural product with low toxicity and few side effects and our research shows that it may be beneficial in treating colorectal cancer," said Randall Holcombe, MD, Professor of Medicine in the Division if Hematology and Oncology at the Icahn School of Medicine at Mount Sinai. "This is an exciting area of research and we look forward to studying the benefits of this compound as an adjunctive treatment in colorectal cancer in humans."
Ron Bentsur, Chief Executive Officer of Keryx, stated, "We are all extremely disappointed with the results of the study. We thank the investigators who participated in what we believe was a well-run study, despite the outcome. We will evaluate whether our Phase 3 study of Perifosine in relapsed/refractory multiple myeloma will continue as planned."
Mr. Bentsur commented further, "With approximately $31 million in cash as of March 31, 2012, and a well-controlled burn rate, we plan to focus our resources on the pending completion of the Zerenex (ferric citrate) long-term Phase 3 study for end stage renal disease (ESRD) patients with hyperphosphatemia, expected in the fourth quarter of 2012, and the New Drug Application (NDA) filing for Zerenex which will hopefully follow shortly thereafter."
We know that, The blackberries, as well as various other Rubus species with mounding or rambling growth habits, are often called brambles. However, this name is not used for those like the raspberry that grow as upright canes, or for trailing or prostrate species such as most dewberries, or various low-growing boreal, arctic, or alpine species. Black raspberries have been also reported to possess antioxidant, anti-cancer, anti-neurodegenerative and anti-inflammatory properties, now the researchers from UIC College of Medicine have looked at the fruit's ability to prevent colon cancer.
The researchers used two strains of mice, Apc1638 and Muc2, which each have a specific gene knocked out, causing the mice to develop either intestinal tumors (in the case of Apc1638) or colitis in the case of Muc2. Colitis is an inflammation of the large intestine that can contribute to the development of colorectal cancer.
Both mouse strains were randomized to be fed either a Western-style, high-risk diet (high in fat and low in calcium and vitamin D) or the same diet supplemented with 10 percent freeze-dried black raspberry powder for 12 weeks.
The researchers found that in both mouse strains the black raspberry-supplemented diet produced a broad range of protective effects in the intestine, colon and rectum and inhibited tumor formation.
In the Apc1638 mice, tumor incidence was reduced by 45 percent and the number of tumors by 60 percent. The researchers found that black raspberries inhibited tumor development by suppressing a protein, known as beta-catenin, which binds to the APC gene.
In the Muc2 mice, tumor incidence and the number of tumors were both reduced by 50 percent, and black raspberries inhibited tumor development by reducing chronic inflammation associated with colitis.
The researchers now hope to obtain funding to begin clinical trials in humans. Because black raspberries not only prevent cancer but also inflammation, they may also protect against other diseases, such as heart disease.
I read an article in the same lines, wherein the researchers attribute the colorectal anticancer activity due to the anthocyanins present
More...