In continuation of my update on formoterol
Circassia Pharmaceuticals plc (“Circassia” or “the Company”; LSE: CIR), a specialty pharmaceutical company focused on respiratory disease, announced the US Food and Drug Administration (FDA) has approved Duaklir for the maintenance treatment of chronic obstructive pulmonary disease (COPD). Duaklir is a fixed-dose combination of the long-acting muscarinic antagonist (LAMA) aclidinium bromide (400 mcg) and long-acting beta-agonist (LABA) formoterol fumarate (12 mcg) administered twice-daily via the breath-actuated inhaler Pressair®. Circassia is on track to launch Duaklir in the United States in the second half of 2019 via its dedicated COPD sales force.
The Duaklir approval is based on a broad clinical database, including data from three phase III studies, ACLIFORM, AUGMENT and AMPLIFY. The label also includes clinical data from the phase IV ASCENT study, which shows aclidinium therapy is effective at reducing COPD exacerbations. As a result, Duaklir® is the only twice-daily LAMA / LABA in the United States with COPD exacerbation data included in its prescribing information.
Steve Harris, Circassia’s Chief Executive, said: “We are delighted with the FDA approval of Duaklir, which we believe will provide a valuable treatment option for the significant number of patients with COPD in the United States. The addition of Duaklir to our portfolio further strengthens our range of marketed respiratory products and we look forward to launching it in the US in the coming months alongside our aclidinium monotherapy, Tudorza, as part of the significant LAMA / LABA market that is predicted to grow rapidly over the coming years.”
Michael Asmus, Circassia’s Vice President, US Medical Affairs, said: “With guidelines recommending combined LAMA and LABA therapy for a number of COPD patient groups, we believe Duaklir will make an important contribution to the treatment of this debilitating disease. Dukalir’s approval is based on a broad clinical database, including data demonstrating a reduction in the risk of COPD exacerbations driven by its aclidinium component, and we look forward to making this new therapeutic option available to patients across the United States.”
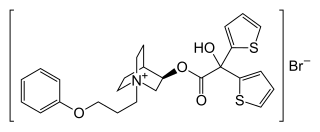 Aclidinium bromide
Aclidinium bromide
https://en.wikipedia.org/wiki/Aclidinium_bromide
https://www.drugbank.ca/drugs/DB00983
