The U.S. Food and Drug Administration today approved Onpattro (patisiran) infusion for the treatment of peripheral nerve disease (polyneuropathy) caused by hereditary transthyretin-mediated amyloidosis (hATTR) in adult patients. This is the first FDA-approved treatment for patients with polyneuropathy caused by hATTR, a rare, debilitating and often fatal genetic disease characterized by the buildup of abnormal amyloid protein in peripheral nerves, the heart and other organs. It is also the first FDA approval of a new class of drugs called small interfering ribonucleic acid (siRNA) treatment.

“This approval is part of a broader wave of advances that allow us to treat disease by actually targeting the root cause, enabling us to arrest or reverse a condition, rather than only being able to slow its progression or treat its symptoms. In this case, the effects of the disease cause a degeneration of the nerves, which can manifest in pain, weakness and loss of mobility,” said FDA Commissioner Scott Gottlieb, M.D. “New technologies like RNA inhibitors, that alter the genetic drivers of a disease, have the potential to transform medicine, so we can better confront and even cure debilitating illnesses. We’re committed to advancing scientific principles that enable the efficient development and review of safe, effective and groundbreaking treatments that have the potential to change patients’ lives.”
RNA acts as a messenger within the body’s cells, carrying instructions from DNA for controlling the synthesis of proteins. RNA interference is a process that occurs naturally within our cells to block how certain genes are expressed. Since its discovery in 1998, scientists have used RNA interference as a tool to investigate gene function and its involvement in health and disease. Researchers at the National Institutes of Health, for example, have used robotic technologies to introduce siRNAs into human cells to individually turn off nearly 22,000 genes.
This new class of drugs, called siRNAs, work by silencing a portion of RNA involved in causing the disease. More specifically, Onpattro encases the siRNA into a lipid nanoparticle to deliver the drug directly into the liver, in an infusion treatment, to alter or halt the production of disease-causing proteins.
Affecting about 50,000 people worldwide, hATTR is a rare condition. It is characterized by the buildup of abnormal deposits of protein fibers called amyloid in the body's organs and tissues, interfering with their normal functioning. These protein deposits most frequently occur in the peripheral nervous system, which can result in a loss of sensation, pain, or immobility in the arms, legs, hands and feet. Amyloid deposits can also affect the functioning of the heart, kidneys, eyes and gastrointestinal tract. Treatment options have generally focused on symptom management.
Onpattro is designed to interfere with RNA production of an abnormal form of the protein transthyretin (TTR). By preventing the production of TTR, the drug can help reduce the accumulation of amyloid deposits in peripheral nerves, improving symptoms and helping patients better manage the condition.
“There has been a long-standing need for a treatment for hereditary transthyretin-mediated amyloidosis polyneuropathy. This unique targeted therapy offers these patients an innovative treatment for their symptoms that directly affects the underlying basis of this disease,” said Billy Dunn, M.D., director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research.
The efficacy of Onpattro was shown in a clinical trial involving 225 patients, 148 of whom were randomly assigned to receive an Onpattro infusion once every three weeks for 18 months, and 77 of whom were randomly assigned to receive a placebo infusion at the same frequency. The patients who received Onpattro had better outcomes on measures of polyneuropathy including muscle strength, sensation (pain, temperature, numbness), reflexes and autonomic symptoms (blood pressure, heart rate, digestion) compared to those receiving the placebo infusions. Onpattro-treated patients also scored better on assessments of walking, nutritional status and the ability to perform activities of daily living.
The most common adverse reactions reported by patients treated with Onpattro are infusion-related reactions including flushing, back pain, nausea, abdominal pain, dyspnea (difficulty breathing) and headache. All patients who participated in the clinical trials received premedication with a corticosteroid, acetaminophen, and antihistamines (H1 and H2 blockers) to reduce the occurrence of infusion-related reactions. Patients may also experience vision problems including dry eyes, blurred vision and eye floaters (vitreous floaters). Onpattro leads to a decrease in serum vitamin A levels, so patients should take a daily Vitamin A supplement at the recommended daily allowance.
The FDA granted this application Fast Track, Priority Review and Breakthrough Therapy designations. Onpattro also received Orphan Drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases.
https://en.wikipedia.org/wiki/Patisiran
----------------------------------------------------------------------------------------
FDA Approves Onpattro (patisiran) Targeted RNA-based Therapy for Polyneuropathy Caused by hATTR
 About S-methylthionine :
About S-methylthionine :


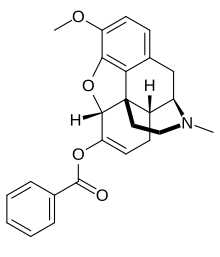 benzhydrocodone
benzhydrocodone 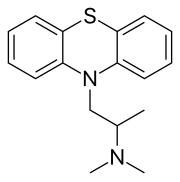 promethazine
promethazine