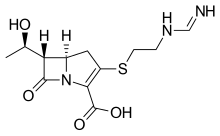
Researchers at the University of Massachusetts Medical School (UMMS) believe, they may have found a new treatment for retinitis pigmentosa (RP), a severe neurodegenerative disease of the retina that ultimately results in blindness. One of the more common retinal degenerative diseases, RP is caused by the death of photoreceptor cells. RP typically manifests in young adulthood as night blindness or a loss of peripheral vision and in many cases progresses to legal blindness by age 40. Dr. Shalesh Kaushal, chair of ophthalmology and associate professor of ophthalmology and cell biology at UMMS, and his team, describe a potential new therapeutic link between valproic acid and RP, which could have tremendous benefits for patients suffering from the disease. In a retrospective study, valproic acid - approved by the FDA to reduce seizures, treat migraines and manage bipolar disorder -- appeared to have an effect in halting vision loss in patients with RP and in many cases resulted in an improved field of vision. Results from this study, in conjunction with prior in vitro data, suggest valproic acid may be an effective treatment for photoreceptor loss associated with RP.
UMass Medical School will be the coordinating site for a $2.1 million, three-year clinical trial funded by the Foundation Fighting Blindness/National Neurovision Research Institute quantifying the potential of valproic acid as a treatment for RP. The clinical trials will build upon Kaushal's work in the retrospective study in which patients were treated off-label with doses of valproic acid ranging from 500mg to 750mg per day over the course of two to six months. Treated at a time when patients normally experience rapid vision loss as a result of RP, five of the seven patients in the study experienced improvement in their field of vision.
"Inflammation and cell death are key components of RP," said Kaushal. "It appears the valproic acid protects photoreceptor cells from this. If our observations can be further substantiated by randomized clinical trials then low dose valproic acid could have tremendous potential to help the thousands of people suffering from RP."
Dr. Kaushal and colleagues, having previously demonstrated the use of the small molecule, retinoid, as a pharmacological agent capable of increasing the yield of properly folded RP rhodopsins, began screening other small molecules for similar attributes. Because of its already known qualities as a potent inhibitor of the inflammatory response pathway and cell death, valproic acid was believed to have a unique profile making it a potential candidate as a retinal disease treatment...


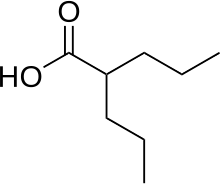 1.valproic acid
1.valproic acid