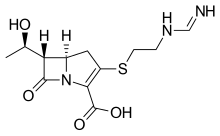
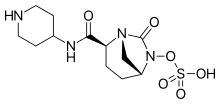
Imipenem (Primaxin) Cilastatin Relebactam
Merck (NYSE:MRK), known as MSD outside the United States and Canada, today announced that the U.S. Food and Drug Administration (FDA) has approved Recarbrio (imipenem, cilastatin, and relebactam) for injection, 1.25 grams, a new combination antibacterial. Recarbrio is indicated in patients 18 years of age and older who have limited or no alternative treatment options, for the treatment of complicated urinary tract infections (cUTI), including pyelonephritis, caused by the following susceptible Gram-negative microorganisms: Enterobacter cloacae, Escherichia coli, Klebsiella aerogenes, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
Recarbrio is also indicated in patients 18 years of age or older who have limited or no alternative treatment options, for the treatment of complicated intra-abdominal infections (cIAI) caused by the following susceptible Gram-negative microorganisms: Bacteroides caccae, Bacteroides fragilis, Bacteroides ovatus, Bacteroides stercoris, Bacteroides thetaiotaomicron, Bacteroides uniformis, Bacteroides vulgatus, Citrobacter freundii, Enterobacter cloacae, Escherichia coli, Fusobacterium nucleatum, Klebsiella aerogenes, Klebsiella oxytoca, Klebsiella pneumoniae, Parabacteroides distasonis and Pseudomonas aeruginosa.
Approval of these indications is based on limited clinical safety and efficacy data for Recarbrio.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Recarbrio and other antibacterial drugs, Recarbrio should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Relebactam received FDA’s Qualified Infectious Disease Product (QIDP) designation for the treatment of cUTI and cIAI. The New Drug Application (NDA) for Recarbrio received Priority Review designation from the FDA. Merck anticipates making Recarbrio available later this year.
Recarbrio is contraindicated in patients with a history of known severe hypersensitivity (severe systemic allergic reaction such as anaphylaxis) to any component of Recarbrio. Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving therapy with beta-lactams. Central nervous system (CNS) adverse reactions, such as seizures, confusional states, and myoclonic activity, have been reported during treatment with imipenem/cilastatin, a component of Recarbrio, especially when recommended dosages of imipenem were exceeded. These reactions have been reported most commonly in patients with CNS disorders (such as brain lesions or a history of seizures) and/or compromised renal function. Concominant use of Recarbrio, with valproic acid or divalproex sodium may increase the risk of breakthrough seizures. Additionally, Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including imipenem/cilastatin plus relebactam and may range in severity from mild diarrhea to fatal colitis. See Important Safety Information below.
Recarbrio is a combination of imipenem/cilastatin and relebactam. Imipenem is a penem antibacterial drug, cilastatin sodium is a renal dehydropeptidase inhibitor, and relebactam is a beta lactamase inhibitor. Cilastatin limits the renal metabolism of imipenem and does not have antibacterial activity. The bactericidal activity of imipenem results from binding to PBP 2 and PBP 1B in Enterobacteriaceae and Pseudomonas aeruginosa and the subsequent inhibition of penicillin binding proteins (PBPs). Inhibition of PBPs leads to the disruption of bacterial cell wall synthesis. Imipenem is stable in the presence of some beta lactamases. Relebactam has no intrinsic antibacterial activity. Relebactam protects imipenem from degradation by certain serine beta lactamases such as Sulhydryl Variable (SHV), Temoneira (TEM), Cefotaximase-Munich (CTX-M), Enterobacter cloacae P99 (P99), Pseudomonas-derived cephalosporinase (PDC), and Klebsiella-pneumoniae carbapenemase (KPC).
“Recarbrio provides an important addition to our toolkit in the ongoing fight against infections caused by certain Gram-negative pathogens,” said Dr. Keith Kaye, professor of medicine and director of research for the division of infectious diseases, University of Michigan Heath System, and a principal investigator in the clinical program. “Recarbrio offers an additional treatment option for patients with cIAI and cUTI who have limited and, in some cases, no alternative therapeutic options.”
“Today’s announcement is a great example of Merck’s longstanding commitment to infectious diseases research and development, as we continue to search for novel ways to approach difficult-to-treat pathogens,” said Dr. Nick Kartsonis, senior vice president, infectious diseases and vaccines, Merck Research Laboratories.
https://en.wikipedia.org/wiki/Imipenem
https://www.drugbank.ca/drugs/DB01597
https://en.wikipedia.org/wiki/Relebactam
