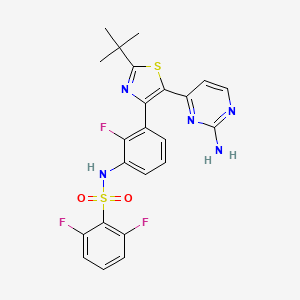
Findings from GlaxoSmithKline (GSK) plc’s Phase 3 clinical study program evaluating single agent therapy with the targeted anti-cancer agents, dabrafenib (below left structure) and trametinib (below right structure), in patients with BRAF V600 mutation positive metastatic melanoma have been released.
Both the BREAK3 study of dabrafenib (BRAF inhibitor) and the METRIC study of trametinib (MEK inhibitor) demonstrated a statistically significant benefit in the length of time patients with BRAF V600 mutation positive advanced or metastatic melanoma lived without progression of their disease or death (Progression Free Survival or PFS) compared to those receiving chemotherapy. Additionally, patients in the METRIC study who received trametinib lived significantly longer (overall survival or OS) than those who received chemotherapy with dacarbazine. OS data are not yet mature in the BREAK3 trial.
“The results from the clinical studies of dabrafenib and trametinib presented at this meeting represent important progress towards understanding how these investigational agents could benefit patients with advanced and metastatic melanoma. Importantly, trametinib is the first MEK inhibitor to demonstrate clinical benefit in a late phase melanoma trial.” said Dr. Rafael Amado, Head of Oncology R&D for GlaxoSmithKline. “We are planning regulatory submissions for dabrafenib and trametinib as single agent therapies and have recently started a Phase 3 program to further investigate the effect of the combination in this disease.”



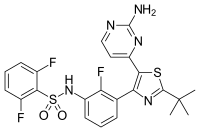


 Trametinib
Trametinib