A subset of lung tumours is exquisitely sensitive to a class of recently approved anti-cancer drugs. Researchers at the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences in Vienna and the Ludwig Institute for Cancer Research in Oxford published this finding in the journal Nature Communications. It opens the way for new clinical trials in a type of cancer considered to be "undruggable" and may lead to a therapy for up to 10% of lung cancer patients.
(Vienna, 6th of December 2016) Lung cancer remains the leading cause of cancer-related deaths worldwide. In contrast to other tumour types, lung tumours present a high number of genomic alterations - this is a consequence of exposure to carcinogenic substances found in tobacco smoke, which is the main cause of lung cancer. About 10% of lung tumours carry mutations in a gene called ATM. However, there are no drugs available in the clinic to treat ATM mutant lung cancer.
With cutting edge high-throughput drug screens that analyse how the genetic makeup of the patient affects their response to drugs, the team of Sebastian Nijman, CeMM Adjunct PI and Group Leader at the Ludwig Institute for Cancer Research in Oxford made a surprising discovery: Cancer cells with ATM mutations are sensitive for drugs that inhibit an enzyme called MEK. The study was published in Nature Communications (DOI: 10.1038/NCOMMS13701)
MEK is part of a biochemical pathway which is responsible for supporting proliferation and survival of the cell, while ATM plays a central role during the DNA damage response. In ATM deficient lung cancer cells, Nijman's team found that MEK inhibition results in cells being unable to keep proliferating and leads to apoptosis. An unexpected finding, as MEK inhibitors have so far been approved for the treatment of a type of skin cancer but not for lung cancer.
"Normally lung cancer cells are resistant to MEK inhibition as they activate compensatory signals," Ferran Fece, one of the two first authors on the study and former PhD student at CeMM, explains. "In contrast, ATM mutant cells fail to do this and subsequently cannot cope with the blocking of MEK and die. We call this type of unexpected drug sensitivity synthetic lethality".
(Vienna, 6th of December 2016) Lung cancer remains the leading cause of cancer-related deaths worldwide. In contrast to other tumour types, lung tumours present a high number of genomic alterations - this is a consequence of exposure to carcinogenic substances found in tobacco smoke, which is the main cause of lung cancer. About 10% of lung tumours carry mutations in a gene called ATM. However, there are no drugs available in the clinic to treat ATM mutant lung cancer.
With cutting edge high-throughput drug screens that analyse how the genetic makeup of the patient affects their response to drugs, the team of Sebastian Nijman, CeMM Adjunct PI and Group Leader at the Ludwig Institute for Cancer Research in Oxford made a surprising discovery: Cancer cells with ATM mutations are sensitive for drugs that inhibit an enzyme called MEK. The study was published in Nature Communications (DOI: 10.1038/NCOMMS13701)
MEK is part of a biochemical pathway which is responsible for supporting proliferation and survival of the cell, while ATM plays a central role during the DNA damage response. In ATM deficient lung cancer cells, Nijman's team found that MEK inhibition results in cells being unable to keep proliferating and leads to apoptosis. An unexpected finding, as MEK inhibitors have so far been approved for the treatment of a type of skin cancer but not for lung cancer.
"Normally lung cancer cells are resistant to MEK inhibition as they activate compensatory signals," Ferran Fece, one of the two first authors on the study and former PhD student at CeMM, explains. "In contrast, ATM mutant cells fail to do this and subsequently cannot cope with the blocking of MEK and die. We call this type of unexpected drug sensitivity synthetic lethality".
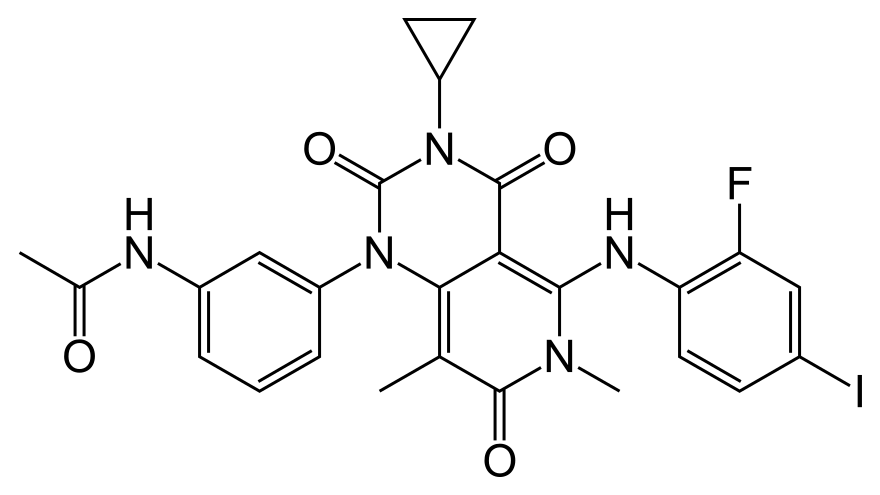 Trametinib
Trametinib
Michal Smida, the other shared first author on the article and former PostDoc at CeMM, adds: "We knew that cancer mutations can lead to extreme sensitivity to some drugs. But finding these cancer Achilles' heels is very difficult as they are difficult to predict and extremely rare. We screened a large number of gene and drug combinations and got lucky."
The study constitutes a substantial contribution for the development of a future precision medicine: ATM mutations could be used as a potential biomarker to stratify lung cancer patients to receive a MEK inhibitor. ATM is found to be mutated in 8-10% of lung adenocarcinomas - given that this type of tumour is among the most prevalent for both men and women worldwide, a significant number of patients could benefit from a MEK inhibitor based treatment.
More : http://www.nature.com/articles/ncomms13701
More : http://www.nature.com/articles/ncomms13701



