Researchers from Case Western Reserve University School of Medicine and the Dana-Farber Cancer Institute have made a fundamental discovery relevant to the understanding and treatment of heart failure -- a leading cause of death worldwide. The team discovered a new molecular pathway responsible for causing heart failure and showed that a first-in-class prototype drug, JQ1, (see structure) blocks this pathway to protect the heart from damage.
In contrast to standard therapies for heart failure, JQ1 works directly within the cell's command center, or nucleus, to prevent damaging stress responses. This groundbreaking research lays the foundation for an entirely new way of treating a diseased heart. The study is published in the August 1 issue of Cell.
"As a practicing cardiologist, it is clear that current heart failure drugs fall alarmingly short for countless patients. Our discovery heralds a brand new class of drugs which work within the cell nucleus and offers promise to millions suffering from this common and lethal disease," said Saptarsi Haldar, MD, senior author on the paper, assistant professor of medicine at Case Western Reserve and cardiologist at University Hospitals Case Medical Center.
Heart failure occurs when the organ's pumping capacity cannot meet the body's needs. Existing drugs, most of which block hormones such as adrenaline at the cell's outer surface, have improved patient survival. Unfortunately, several clinical studies have demonstrated that heart failure patients taking these hormone-blocking drugs still succumb to high rates of hospitalization and death. Leveraging a new approach, the research team turned their attention from the cell's periphery to the nucleus -- the very place that unleashes sweeping damage-control responses which, if left unchecked, ultimately destroy the heart.
The team found that a new family of genes, called BET bromodomains, cause heart failure because they drive hyperactive stress responses in the nucleus. Prior research linking BET bromodomains to cancer prompted the laboratory of James Bradner, MD, the paper's senior author and a researcher at the Dana-Farber, to develop a direct-acting BET inhibitor, called JQ1. In models of cancer, JQ1 functions to turn off key cancer-causing genes occasionally prompting cancer cells to "forget" they are cancer. In models of heart failure, JQ1 silences genetic actions causing enlargement of and damage to the heart even in the face of overwhelming stress.
"While it's been known for many years that the nucleus goes awry in heart failure, potential therapeutic targets residing in this part of the cell are often dubbed as 'undruggable' given their lack of pharmacological accessibility," said Jonathan Brown, MD, cardiologist at Brigham and Women's Hospital and co-first author on the paper. "Our work with JQ1 in pre-clinical models shows that this can be achieved successfully and safely."


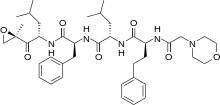 Carfilzomib
Carfilzomib 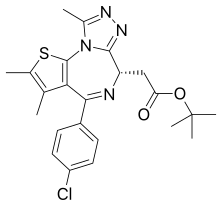 JQ1
JQ1  ABT-199
ABT-199