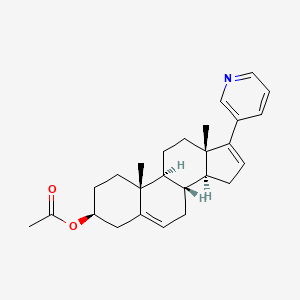
We know that
, Abiraterone (discovered and developed at the
Institute of Cancer Research in London
, see structure) is a drug under investigation for use in hormone-refractory prostate cancer (
prostate cancer not responding to treatment with antiandrogens). Abiraterone
acts by blocking the formation of testosterone by inhibiting CYP17A1 (CYP450c17), an enzyme also known as 17α-hydroxylase/17,20 lyase.
This enzyme is involved in the formation of DHEA and androstenedione, which may ultimately be metabolized into testosterone.
The latest trial, which was led by the ICR and the Royal Marsden NHS Foundation Trust, is the first to investigate the drug in men with such advanced prostate cancer.
A total of 47 men were recruited for the trial, all of whom had late-stage castration-resistant prostate cancer, which means that their disease was advanced and their tumors were no longer responsive to androgen deprivation therapy. In almost all cases, the men's cancer had spread to their bones. All of the participants had already received hormone therapy and the chemotherapy drug docetaxel, but were no longer responding to those treatments. By the end of the study period, researchers found that around three-quarters of men had experienced a drop in levels of prostate specific antigen (PSA), which is often raised in men with prostate cancer and can be used to measure disease activity.
In around half of the men, PSA levels fell by at least 50 per cent, while three-quarters of participants also had a drop in the number of tumor cells circulating in their blood. Three years after the start of the trial, five of the patients were still taking abiraterone and benefitting from the treatment. Lead researcher Dr Alison Reid, also from the ICR and the Royal Marsden, noted that "abiraterone shrank or stabilised men's cancers for an average of almost six months, which is a very impressive result with only mild side-effects".
Though the initial results are exciting, the researchers conclude that there's a lot more work needed to establish what abiraterone's place will be in treating men with prostate cancer....
Ref :http://info.cancerresearchuk.org/news/archive/cancernews/2010-02-16-New-drug-shows-promise-for-advanced-prostate-cancer-patients