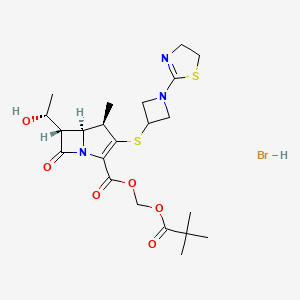
Arcutis Biotherapeutics, Inc. In continuation of my update on roflumilast has submitted a new drug application (NDA) to the U.S. Food and Drug Administration (FDA) for roflumilast cream for the treatment of mild-to-severe plaque psoriasis.

Roflumilast cream (ARQ-151) is a once-daily topical formulation of roflumilast, a highly potent and selective inhibitor of phosphodiesterase type 4 (PDE4), an enzyme that drives overactive immune responses. In clinical trials, roflumilast cream demonstrated robust efficacy coupled with favorable safety and tolerability that, if approved, would enable chronic use across the body, without many of the local tolerability issues associated with alternative treatments.
“Today is a critical milestone for Arcutis in our efforts to bring innovative treatments to dermatologists and their patients, and is a reflection of our deep dermatology expertise,” said Frank Watanabe, President and CEO of Arcutis. “Individuals with plaque psoriasis currently do not have topical treatment options that offer a combination of good tolerability and the ability to be used for long periods of time, and that can be used on all parts of the body. If approved, roflumilast cream will be the first and only topical PDE4 inhibitor approved for psoriasis and an important non-steroidal treatment option for the millions of individuals struggling with plaque psoriasis. I want to thank the Arcutis team, as well as the clinical investigators, patients, and partners, for helping us reach this important milestone.”
Psoriasis is a common, non-contagious, immune-mediated skin disease that affects more than 3% of the U.S. population. The majority of patients develop “plaques,” or raised, red areas of skin covered with a silver or white layer of dead skin cells. Psoriatic plaques are often itchy and sometimes painful, and can appear on any area of the body. Plaques in certain anatomical areas present particular treatment challenges, including the face, elbows and knees, scalp, and areas where two skin areas may touch or rub together.
Topical treatments are the mainstay of therapy for the vast majority of psoriasis patients, particularly those with mild-to-moderate disease, as well as many moderate-to-severe patients who use topicals in combination with other treatments. However, existing topical treatments often force physicians and patients to make difficult trade-offs between tolerability and long-term use, requiring the use of multiple products or complicated treatment schedules. Roflumilast cream has been designed to address the challenges posed to dermatologists and patients by existing topical therapies and aims to simplify the overall management of plaque psoriasis.
Arcutis’ submission is supported by positive data from Arcutis’ pivotal Phase 3 program. The DERMIS 1 and DERMIS 2 (Trials of PDE4 inhibition with Roflumilast for the Management of plaque PsoriasIS” One and Two) were identical Phase 3 randomized, parallel, double-blind, vehicle-controlled, multi-national, multi-center studies to evaluate the safety and efficacy of roflumilast cream 0.3%. Roflumilast met its primary endpoint and had an ‘IGA Success’ rate of 42.4% compared to a vehicle rate of 6.1% (P<0.0001), and 37.5% compared to a vehicle rate of 6.9% (P<0.0001), in DERMIS 1 and 2 respectively. Roflumilast cream 0.3% also demonstrated statistically significant improvements over vehicle on key secondary endpoints, including on Intertriginous IGA (I-IGA) Success, Psoriasis Area Severity Index-75 (PASI-75), reductions in itch as measured by the Worst Itch-Numerical Rating Scale (WI-NRS), and patient perceptions of symptoms as measured by the Psoriasis Symptoms Diary (PSD). In trials, roflumilast cream was generally well-tolerated with a favorable safety and tolerability profile.
https://en.wikipedia.org/wiki/Roflumilast