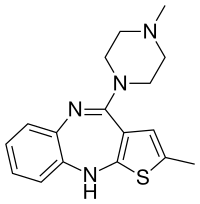
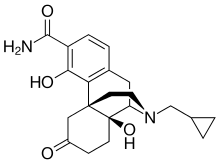
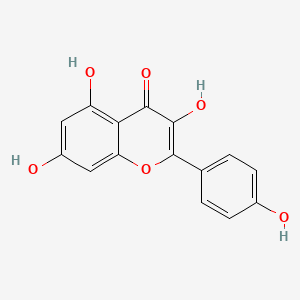
In continuation of my update on kaempferol, myricetin and quercetin
kaempferol
myricetin
Quercetin
People who eat or drink more foods with the antioxidant flavonol, which is found in nearly all fruits and vegetables as well as tea, may be less likely to develop Alzheimer's dementia years later, according to a study published in the January 29, 2020, online issue of Neurology, the medical journal of the American Academy of Neurology.
"More research is needed to confirm these results, but these are promising findings," said study author Thomas M. Holland, MD, of Rush University in Chicago. "Eating more fruits and vegetables and drinking more tea could be a fairly inexpensive and easy way for people to help stave off Alzheimer's dementia. With the elderly population increasing worldwide, any decrease in the number of people with this devastating disease, or even delaying it for a few years, could have an enormous benefit on public health."
Flavonols are a type of flavonoid, a group of phytochemicals found in plant pigments known for its beneficial effects on health.
The study involved 921 people with an average age of 81 who did not have Alzheimer's dementia. The people filled out a questionnaire each year on how often they ate certain foods. They were also asked about other factors, such as their level of education, how much time they spent doing physical activities and how much time they spent doing mentally engaging activities such as reading and playing games.
The people were tested yearly to see if they had developed Alzheimer's dementia. They were followed for an average of six years. The researchers used various tests to determine that 220 people developed Alzheimer's dementia during the study.
The people were divided into five groups based on how much flavonol they had in their diet. The average amount of flavonol intake in US adults is about 16 to 20 milligrams per day. In the study, the lowest group had intake of about 5.3 mg per day and the highest group consumed an average of 15.3 mg per day.
The study found that people in the highest group were 48 percent less likely to later develop Alzheimer's dementia than the people in the lowest group after adjusting for genetic predisposition and demographic and lifestyle factors. Of the 186 people in the highest group, 28 people, or 15 percent, developed Alzheimer's dementia, compared to 54 people, or 30 percent, of the 182 people in the lowest group.
The results were the same after researchers adjusted for other factors that could affect the risk of Alzheimer's dementia, such as, diabetes, previous heart attack, stroke and high blood pressure.
The study also broke the flavonols down into four types: isorhamnetin, kaempferol, myricetin and quercetin. The top food contributors for each category were: pears, olive oil, wine and tomato sauce for isorhamnetin; kale, beans, tea, spinach and broccoli for kaempferol; tea, wine, kale, oranges and tomatoes for myricetin; and tomatoes, kale, apples and tea for quercetin.
People who had high intake of isorhamnetin were 38 percent less likely to develop Alzheimer's. Those with high intake of kaempferol were 51 percent less likely to develop dementia. And those with high intake of myricetin were also 38 percent less likely to develop dementia. Quercetin was not tied to a lower risk of Alzheimer's dementia.
Holland noted that the study shows an association between dietary flavonols and Alzheimer's risk but does not prove that flavonols directly cause a reduction in disease risk.
Other limitations of the study are that the food frequency questionnaire, although valid, was self-reported, so people may not accurately remember what they eat, and the majority of participants were white people, so the results may not reflect the general population.
https://en.wikipedia.org/wiki/Myricetin
https://en.wikipedia.org/wiki/Quercetin
https://medicalxpress.com/news/2019-07-high-hemoglobin-linked-dementia.html







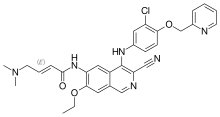 Everolimus
Everolimus