According to a handful of recent studies, okra may reduce symptoms of diabetes - a group of diseases that includes type 1 diabetes, type 2 diabetes, and gestational diabetes.
Diabetes claimed the lives of 75,578 Americans in 2013, according to the United States Centers for Disease Control and Prevention (CDC). In 2014,8.5 percent of adults worldwide had the condition, the World Health Organization (WHO) report. By 2030, diabetes may be the seventh leading cause of death.
A number of factors increase a person's risk of developing diabetes, including a family history of the disease. Lifestyle factors also play a role, so doctors routinely recommend diet changes and increased exercise to reduce blood sugar levels.
Okra may help reduce blood sugar levels in some people with diabetes. Research into the effects of this seedy vegetable is still in the early stages, but the results are promising.
Okra thrives in temperate climates, producing large hibiscus-like flowers that eventually give rise to green seed pods. It is a member of the mallow family, which includes a number of other popular plants, including hibiscus, cocoa, and cotton.
Scientifically known as Abelmoschus esculentus, okra may have been grown as long ago as 2000 BCE in Egypt.
Okra's flavor is mild, and the entire seed pod can be eaten. This vegetable-like fruit also has a long history in traditional medicine.
Kew Royal Botanic Gardens report that in Eastern traditional medicine, okra leaves and fruit were used as pain relievers, moisturizers, and to treat urinary disorders. In Congolese medicine, okra is used to encourage a safe delivery during childbirth.
Can okra help with symptoms of diabetes?
Diabetes can often be well-managed with increasing a hormone called insulin and other medical therapies. However, some people with diabetes wish to avoid regular insulin injections. Others experience blood sugar dips and other unpleasant side effects, and diabetes medications do not work for everyone.
The possibility that a readily available seed pod could help control diabetes is an exciting one. But there is no evidence yet that okra can cure diabetes. So far, the research on okra has only looked at its effects on animals. Human bodies are similar to animals, but not all research on animals has worked out in humans.
Increased absorption of sugar by muscles
A 2005 study published in Planta Medica investigated the effects of okra on rats with diabetes. A substance called myricetin is present in okra and some other foods, including red wine and tea.
Researchers isolated myricetin from okra, then administered it to the rat. The treatment increased absorption of sugar in the rats' muscles, lowering their blood sugar.
A 2012 Food Science and Human Wellness review points to a number of other laboratory and animal studies that have linked myricetin to lower blood sugar. The study argues that myricetin may also reduce other risk factors for diabetes.
Reduction in blood sugar spikes after eating
A 2011 study published in ISRN Pharmaceutics found a link between okra and decreased blood sugar spikes after eating.
Researchers fed rats liquid sugar as well as purified okra through a feeding tube. Rats who consumed the okra experienced a reduction in blood sugar spikes after feeding. The study's authors think this is because the okra blocked the absorption of sugar in the intestines.
The study also explored possible interactions between okra and metformin, a drug that can reduce blood sugar in type 2 diabetes. Okra appeared to also block absorption of metformin. This suggests that okra could reduce the effectiveness of metformin, and should therefore not be eaten at the same time as the drug.
Lower blood sugar levels
A 2011 study published in the Journal of Pharmacy and Bioallied Sciences points to a link between eating okra and lower blood sugar. The researchers allowed the blood sugar of rats with diabetes to stay level for 14 days. They then gave the rats powdered okra peel extracts and seeds dosages of up to 2,000 milligrams per kilogram of body weight.
There were no poisonous effects linked with these relatively high doses of okra. The rats that ate okra had reduced blood sugar levels after up to 28 days of eating okra. The study ended on day 28, so it is unclear if the effects on blood sugar levels would have lasted longer.
Considerations for using okra
Few studies have linked okra to negative side effects, but some negative side effects are possible:
- Okra may make the drug metformin less effective.
- Okra is high in substances known as oxalates. Oxalates may increase the risk of kidney stones in people vulnerable to kidney stones.
- Okra can contain bacteria, pesticides, and other dangerous substances if it is not thoroughly washed. People should never consume rotten okra, frozen okra that is past its expiration date, or okra that has not been thoroughly washed.
- People with an okra allergy should not consume okra. Those with an allergy to other plants in the mallow family, such as hibiscus or cotton, may also be allergic to okra.
- Even if okra proves to be ineffective in fighting diabetes, it remains a safe snack for people with diabetes. A single serving of 100 grams contains just 30 calories, but offers a number of nutritional benefits:
- Okra contains no saturated fats or cholesterol
- Okra is rich in fiber, containing 9 percent of the recommended daily value (RDV)
- Okra contains 8 percent of the RDV of calcium, 43 percent of the RDV of manganese, 10 percent of the RDV of iron and copper, and 44 percent of the RDV of vitamin K
Okra is rich in protective substances known as antioxidants, including myricetin. According to the National Center for Complementary and Integrative Health, antioxidants may reduce oxidative stress, a process that damages cells in the body. Oxidative stress plays a role in the development of diabetes, as well as diseases such as:- Parkinson's disease
- Alzheimer's disease
- Cataracts
- Macular degeneration
- Heart and blood vessel disease
- Cancer
In addition to its antioxidant benefits, okra may also reduce tiredness. A 2015 study published in Nutrients found that substances found in okra seeds known as polyphenols and flavonoids could reduce fatigue.



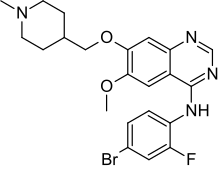

 CX546
CX546