In continuation of my update on phenanthriplatin
In a pair of firsts, researchers at Case Western Reserve University and Massachusetts Institute of Technology have shown that the drug candidate phenanthriplatin can be more effective than an approved drug in vivo, and that a plant-virus-based carrier successfully delivers a drug in vivo.
 phenanthriplatin
phenanthriplatin
Triple-negative breast cancer tumors of mice treated with the phenanthriplatin -carrying nanoparticles were four times smaller than those treated either with cisplatin, a common and related chemotherapy drug, or free phenanthriplatin injected intravenously into circulation.
The scientists believe the work, reported in the journal ACS Nano, is a promising step toward clinical trials.
"We may have found the perfect carrier for this particular drug candidate," said Nicole Steinmetz, an assistant professor of biomedical engineering at Case Western Reserve, who has spent 10 years studying the use of plant viruses for medical purposes.
She teamed with Stephen J. Lippard, Arthur Amos Noyes Professor of chemistry at MIT, and an expert in biological interactions involving platinum-based chemotherapies.
Platinum-based drugs are used to treat more than half of cancer patients receiving chemotherapy. Two of the most commonly used drugs are cisplatin and carboplatin. They form bifunctional cross-links with DNA in cancer cells, which block the DNA from transcribing genes and result in cell death, Lippard explained.
Despite widespread use, cisplatin has been shown to cure only testicular cancer, and many cancers have or develop immunity to the drug.
Lippard's lab altered cisplatin by replacing a chloride ion with phenanthridine and found that the new molecule also binds to DNA. Instead of forming cross-links, however, phenanthriplatin binds to a single site but still blocks transcription.
In fact, his lab found that phenanthriplatin is up to 40 times more potent than traditional platins when tested directly against cancer cells of lung, breast, bone and other tissues. The molecule also appears to avoid defense mechanisms that convey resistance.
But when injected into mouse models of cancer, the drug candidate performed no better than standard platins.
Lippard realized phenanthriplatin wasn't reaching its target. He had a drug delivery problem.
He found a potential solution while visiting Case Western Reserve's campus and heard Steinmetz explain her work investigating tobacco mosaic virus (TMV) for drug delivery more than a year ago.
"I envisioned that TMV would be the perfect vehicle," Lippard said. "So we had a beer and formed a collaboration."
The long, thin tobacco mosaic virus nanoparticles are naturals for delivering the drug candidate into tumors, said Steinmetz, who was appointed by the Case Western Reserve School of Medicine.
The virus particles, which won't infect humans, are hollow. A central tube about 4 nanometers in diameter runs the length of the shell and the lining carries a negative charge.
Phenanthriplatin is about 1 nanometer across and, when treated with silver nitrate, has a strong positive charge. It readily enters and binds to the central lining.
The elongated shape of the nanoparticle causes it to tumble along the margins of blood vessels, remain unnoticed by immune cells and pass through the leaky vasculature of tumors and accumulate inside. Little healthy tissue is exposed to the toxic drug.
Inside tumors, the nanoparticles gather inside the lysosomal compartments of cancer cells, where they are, in essence, digested. The pH is much lower than in the circulating blood, Steinmetz explained. The shell deteriorates and releases phenanthriplatin.
The shell is broken down into proteins and cleared through metabolic or natural cellular processes within a day while the drug candidate starts blocking transcription, leading to greater amounts of cell death through apoptosis than cross-linking platins.
The researchers say delivery of the phenanthriplatin into the tumor led to its improved performance over cisplatin or free phenanthriplatin.
Lippard and Steinmetz continue to collaborate, investigating use of this system to deliver other drugs or drug candidates, use in other types of cancers, the addition of agents on the exterior of the shell to increase accumulation inside tumors and more.



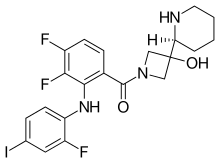 Cobimetinib
Cobimetinib 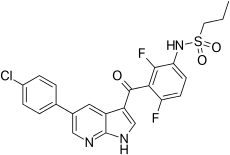 vemurafenib
vemurafenib