A promising new compound appears to impede a process that fuels breast cancer in mice, a discovery that could have implications in the treatment of a host of cancers.
On top of short-circuiting the proliferation of cancer cells, a new agent that the researchers called Fasnall also contributed to the death of existing cancer cells, according to scientists from The Ohio State University and Duke University.
The mice injected with Fasnall survived for an average of 63 days, more than double the lifespan of the mice in the control group. After three weeks, tumors in the mice that received Fasnall were about two-thirds the size of those in the control group, the researchers report in a study published in the journal Cell Chemical Biology.
When researchers tried Fasnall alongside the chemotherapy drug carboplatin, they saw tumors shrink and survival increase more than with either agent by itself.
The study focused on mice with HER2-positive breast cancer, which is responsible for about one in five breast cancer diagnoses in women. But because of the critical role of an enzyme called fatty acid synthase in a variety of cancers, this work could have much broader implications, said Ohio State's Jesse Kwiek, an associate professor of microbiology and microbial infection and immunity.
The discovery, five years in the making, was speedy by drug development standards, he said.
"We started with an idea and got it to work in a mouse in a relatively short amount of time," Kwiek said.
"It's a promising starting point."
He and Duke's Timothy Haystead, a cancer biologist who co-led the study, are seeking a patent.
Fasnall inhibits the normal activity of fatty acid synthase, which regulates cell growth and proliferation.
"Tumor cells are quite dependent on that enzyme as a fuel source for survival," Haystead said. "If you nail this target, you're selectively striking the tumor rather than normal cells. And not only do you starve the tumor cell of its energy source, but also trigger changes that convince the cell to essentially kill itself."
Scientists exploring opportunities to close the doors on cancer growth have known for some time that many solid tumors depend on fatty acid synthase. Most other cells in the body are either less reliant on the enzyme, or don't need it at all, reducing the chances that harmful side effects would overshadow benefits.
All of that makes for an obvious, but thus far tricky, target for cancer fighters in the lab.
"It's always this balance where you try to identify molecules that are more important to the malignancy than to the host," Kwiek said. "You're looking for these little tweaks - little advantages."
In this case, that means interrupting fatty acid synthesis, effectively robbing the cancer of a molecule it needs in order to grow.
"Fasnall inhibits the ability of this enzyme to make palmitic acid, a molecule important for many cellular processes," Kwiek said.
And when the enzyme isn't doing its normal job, it appears to be diverted elsewhere - to a place where it has the added benefit of provoking the programmed death of cancer cells.
Before the mouse study, the research team sifted through a pool of 3,400 molecules looking for one that was efficient at knocking out fatty acid synthase in pig mammary glands without causing much residual harm. They first narrowed the field to about 1,300, then to 13 strong contenders.
Then the researchers examined each of the 13 finalists' activity within a cell. Fasnall rose to the top. Not only did it inhibit the tumor-fueling activity, it didn't take much of the compound for that to happen, which lowered the chances it would be toxic to the mice.
The discovery stemmed from an effort to look for novel treatments for cancer and HIV. Fatty acid synthase, disrupted by Fasnall, plays a role in both. The research team has not yet published results on their HIV work.
"Cancer is uncontrolled cell division, and fatty acid synthase helps make the raw materials that make the cells divide," Kwiek said.
The mice in the study showed no signs of major side effects, such as weight gain or loss or significant changes in liver enzymes, he said.
It appears the dose could be increased from the amount used in this research and that could produce more dramatic results, Kwiek said.
Fasnall needs more testing in animals before it can be employed in human studies, the researchers said. Other fatty acid inhibitors are under review, but thus far none has made it to market and none operates in precisely the way Fasnall does, Kwiek said.
The mechanism by which it works is less likely to run up against drug resistance in the cancer cells than some other approaches, Haystead said.
Its potential as one element of a cancer treatment cocktail is attractive, because it's possible Fasnall would offset the need for high doses of potent treatments that come with serious side effects, Haystead said.
"There are a huge gamut of implications and some may be better than others. Our job now is to sort of move this molecule down the clinical path," he said.
The researchers caution that this is the first, albeit big, step in a process that would take years if all goes well.
"This is just a mouse model of a single cancer," Kwiek said.
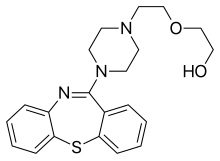 quetiapine.
quetiapine.
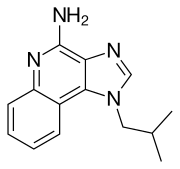



 Lenvatinib
Lenvatinib