In continuation of my updates on Dexamethasone, Plitidepsin and Bortezomib
PharmaMar (MSE:PHM) announces the positive results from a Phase I study of plitidepsin in combination with bortezomib and dexamethasone in patients with relapsed and/or refractory multiple myeloma. Dr María Victoria Mateos, MD of the Hematological Department of the University Hospital of Salamanca, Spain, the principal investigator of the study, will present the results in an oral session on June 3rd, 2016 during the 52nd Congress of the American Society of the Clinical Oncology (ASCO), taking place in Chicago (USA), June 3 - 7.
The primary objective of this 20-patient study was to identify the recommended dose for the triple combination (dexamethasone / bortezomib / plitidepsin) administered every four weeks. Efficacy and the safety profile were also evaluated. The overall response rate (ORR) was 56%, including very good partial responses (VGPR) in 33% of the patients and a remarkable partial remission in one triple refractory patient. The median progression free survival (PFS) was 8.3 months. Additionally, 90% of the patients showed a DOR of 6 months or more and clinical benefit was observed in 72% of the patients.
Dose limiting toxicities were not seen in any of the evaluated patients; therefore, the full dose of plitidepsin and bortezomib when used alone were established as the recommended dose for the triple combination. The treatment was well tolerated. The hematological toxicity was manageable and the non-hematological toxicity was in general mild, with the exception of one case of creatinine increase.
Out of the 20 patients that participated in the study, 10 are still under the treatment. The median age was 65. All patients had relapsed after previously receiving, on average, 3.5 therapeutic regimens (range 1-10). Forty-five percent of these patients had been subject to a hematopoietic stem cell transplant (8 autologous, 1 allogeneic). Of the 18 patients evaluable for efficacy, 83% (15 patients) had previously received bortezomib and lenalidomide. One was refractory to bortezomib and seven to lenalidomide.
In abstract #8006, Dr María Victoria Mateos and her team explain that despite the recent progress in the treatment of multiple myeloma due to the introduction of proteasome inhibitors (PIs), the new immunomodulatory drugs (IMIDs), and monoclonal antibodies, the illness is still incurable. Therefore, active compounds with novel mechanisms of action and adequate safety profile are needed. Plitidepsin targets the eukaryotic Elongation Factor eEF1A2, an overexpressed protein in multiple myeloma that contributes to its pathogenesis. The positive results from this study will be added to the already extensive data package from Phase II and Phase III trials, where plitidepsin has shown activity and a favorable safety profile in combination with dexamethasone.

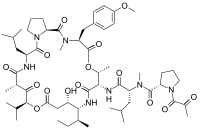
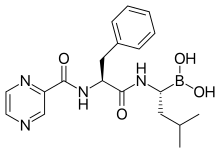
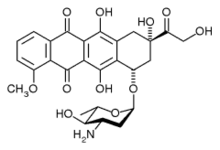 epirubicin
epirubicin letrozole
letrozole 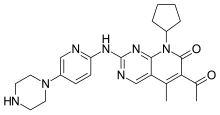 palbociclib
palbociclib