New data presented today confirms that a novel first-in-class treatment for Hepatitis B, called NVR 3-778, is well-tolerated and can reduce levels of the virus' genetic material in the body when combined with pegylated interferon after four weeks of treatment. The updated Phase 1b trial results were presented today at The International Liver CongressTM 2016 in Barcelona, Spain.
NVR 3-778 is a first-in-class HBV capsid assembly inhibitor which modulates the function of the core protein. This protein plays an essential role in viral replication and persistence of the virus.
Approximately 14 million people within the World Health Organization European region are chronically infected with Hepatitis B.1 There are several medicines that are effective at suppressing the virus over many years, slowing down damage to the liver, and allowing the body to repair itself.2 However, it is unusual for these treatments to clear the virus permanently.3
"Previous Phase 1 results with NVR 3-778 have shown reduction in HBV viral load," said Dr Man-Fung Yuen of Queen Mary Hospital, University of Hong Kong, and lead author of the study. "It is promising to see that the combination of NVR 3-778 with pegylated interferon produces responses that are greater than those seen with either monotherapy."
The international Phase 1b study was conducted in 64 patients who had not previously received any treatment for Hepatitis B. There were six dosing cohorts in the study: 100mg daily, 200mg daily, 400mg daily, 600mg twice a day, or 600mg twice a day combined with pegylated interferon, and finally pegylated interferon combined with placebo. Treatment was given for a total of 28 days.
The results demonstrated that NVR 3-778 was well tolerated in all cohorts with no discontinuations. Most adverse events were mild and not attributed to the study drug. Dose-related reductions in HBV DNA were observed, the largest of which was in the NVR 3-778 and pegylated interferon combination (1.97 log IU/mL). Using NVR 3-778 alone, the HBV DNA reduction was 1.72 log10 in the 600 mg BD group, and in the pegylated interferon alone group the HBV DNA reduction was 1.06 log10. Study results also indicated early reductions in levels of HBeAg (a sign that the virus is actively replicating in the body and that the infection is worse) across all groups, which were greatest in the NVR 3-778 group.
"The results from this study are certainly interesting and promising for the treatment of patients with Hepatitis B,'" said Professor Frank Tacke, EASL Governing Board member. "The medical community is always on the look-out for treatments which can cure this condition, as opposed to simply suppressing the replication of the virus. More research is needed to confirm whether NVR 3-778 could really change the treatment paradigm."



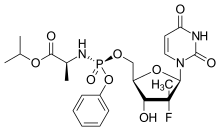 Sofosbuvir
Sofosbuvir