Researchers from UCLA and Shanghai Jiao Tong University have made an important step toward a substantially faster and more effective treatment for tuberculosis, which infects some 10 million people and causes 1.5 million deaths each year.
Combination therapy, which utilizes a series of drugs, is a clinical standard for many major diseases. However, the number of potential combinations of different drugs and dose levels can be in the billions, making the prospect of choosing the best one seem daunting.
The research was published in the Proceedings of the National Academy of Sciences.
In the study, researchers used a technique called feedback system control, which was developed at UCLA, to study cells infected with the bacteria that cause tuberculosis. They quickly narrowed combinations of 14 different tuberculosis drugs with five different doses -- resulting in 6 billion possibilities -- into several promising combination treatments that kill the bacteria that cause tuberculosis much faster than the standard regimen used to treat tuberculosis.
"Designing a drug combination with optimized drug-dose ratios has, until now, been virtually impossible," said Chih-Ming Ho, the study's principal investigator and the Ben Rich-Lockheed Martin Chair Professor at UCLA's Henry Samueli School of Engineering and Applied Science. "Feedback system control technology demonstrated it can pinpoint these best possible ratios for a wide spectrum of diseases."
"If our findings are confirmed in human studies, the new drug regimens that we have identified should dramatically shorten the time needed to treat tuberculosis," said Dr. Marcus Horwitz, a senior author on the research and a distinguished professor of medicine and microbiology, immunology and molecular genetics at the UCLA David Geffen School of Medicine. "This will increase the likelihood of successful treatment and decrease the likelihood of patients developing drug-resistant tuberculosis. A highly successful and rapid treatment may hasten the eventual eradication of tuberculosis."
New drug regimens could significantly improve treatment for tuberculosis: Researchers from UCLA and Shanghai Jiao Tong University have made an important step toward a substantially faster and more effective treatment for tuberculosis, which infects some 10 million people and causes 1.5 million deaths each year.




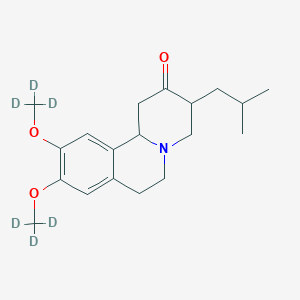

 (Dapsone)
(Dapsone)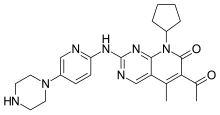 Palbociclib
Palbociclib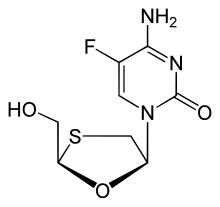 Emtricitabine
Emtricitabine  Rilpivirine
Rilpivirine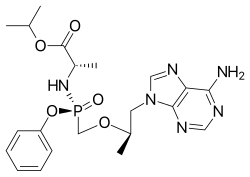 Tenofovir alafenamide
Tenofovir alafenamide
