In continuation of my update on Dolutegravir & Lamivudine

https://en.wikipedia.org/wiki/Dolutegravir
https://en.wikipedia.org/wiki/Lamivudine

Dolutegravir (DTG)

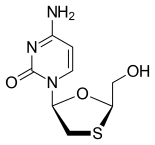
Lamivudine, commonly called 3TC
ViiV Healthcare announced the US Food and Drug Administration (FDA) approval of Dovato, a complete, once-daily, single-tablet regimen of dolutegravir (DTG) 50 mg and lamivudine (3TC) 300 mg for the treatment of HIV-1 infection in adults with no antiretroviral (ARV) treatment history and with no known resistance to either DTG or 3TC. Dovato, a two-drug regimen (2DR), reduces exposure to the number of ARVs from the start of treatment, while still maintaining the efficacy and high barrier to resistance of a traditional DTG-based three-drug regimen.
Deborah Waterhouse, CEO, ViiV Healthcare, said: “Building on our innovative portfolio of medicines, Dovato is powered by dolutegravir, an antiretroviral included in multiple combination therapies and the most prescribed integrase inhibitor in the world, 2 coupled with the established profile of lamivudine. With Dovato, the first complete, single-tablet, two-drug regimen for treatment-naïve adults, ViiV Healthcare is delivering what patients are requesting—a chance to treat their HIV-1 infection with as few drugs as possible, marking a significant step in HIV treatment.”
The approval of Dovato is supported by the landmark global GEMINI 1 and 2 studies that included more than 1,400 HIV-1 infected adults. In these studies, DTG + 3TC demonstrated non-inferiority based on plasma HIV-1 RNA <50 copies per milliliter (c/mL), a standard measure of HIV-1 control, at Week 48 when compared to a three-drug regimen of DTG and two nucleoside reverse transcriptase inhibitors (NRTIs), tenofovir disoproxil fumarate/emtricitabine (TDF/FTC), in treatment-naïve, HIV-1 infected adults. The safety results for DTG + 3TC seen in GEMINI 1 and 2 were consistent with the product labelling for DTG and 3TC. No patient who experienced virologic failure in either treatment arm developed treatment-emergent resistance.
Pedro Cahn, principal investigator for the GEMINI study program said: “People are now living longer with HIV and will spend a lifetime taking drugs to suppress their virus. The approval of the fixed dose combination of dolutegravir and lamivudine, a complete, single-tablet, two-drug regimen, marks a pivotal moment in the treatment of HIV-1. Treatment-naïve people living with the virus have a powerful option that delivers non-inferior efficacy to a dolutegravir-based three-drug regimen, allowing them to take fewer ARVs and get and remain suppressed.”
Jeff Berry, Test Positive Aware Network (TPAN), said: “The approval of Dovato is a welcome paradigm shift, as it brings an innovative treatment approach to newly diagnosed adults with HIV-1. By exposing patients to fewer drugs at the start of treatment, the hope is to help address concerns arising from overall management of prolonged ARV therapy.”
DTG/3TC as a complete, once-daily, single-tablet, two-drug regimen for HIV-1 therapy is currently under review by the European Medicines Agency (EMA) and regulatory authorities in Canada, Australia, Switzerland, and South Africa and several additional submissions are planned throughout 2019.
About Dovato (dolutegravir/lamivudine)
Dovato is approved as a complete regimen for the treatment of HIV-1 infection in adults with no known antiretroviral treatment history and with no known substitutions associated with resistance to either dolutegravir or lamivudine. Dovato is a once-daily, single-tablet, two-drug regimen that combines the integrase strand transfer inhibitor (INSTI) dolutegravir (Tivicay, 50 mg) with the nucleoside analogue reverse transcriptase inhibitor (NRTI) lamivudine (Epivir, 300 mg).
Like a DTG-based three-drug regimen, Dovato uses only two drugs to inhibit the viral cycle at two different sites. INSTIs, like dolutegravir, inhibit HIV replication by preventing the viral DNA from integrating into the genetic material of human immune cells (T-cells). This step is essential in the HIV replication cycle and is also responsible for establishing chronic infection. Lamivudine is an NRTI that works by interfering with the conversion of viral RNA into DNA which in turn stops the virus from multiplying.
https://en.wikipedia.org/wiki/Lamivudine

 rilpivirine
rilpivirine 

