Friday, December 5, 2025
FDA Approves Yeztugo (lenacapavir) as the First and Only HIV Prevention Option Offering 6 Months of Protection
Monday, January 24, 2022
FDA Approves Apretude
Cabotegravir long-acting for PrEP is provided as an injection given as few as six times per year and is initiated with a single 600 mg (3-ml) injection given one month apart for two consecutive months. After the second initiation injection, the recommended continuation injection dose is a single 600 mg (3-ml) injection given every two months. Vocabria (cabotegravir oral tablets) may be administered for approximately one month before initiating the first injection to assess the tolerability of the medicine.
Deborah Waterhouse, CEO, ViiV Healthcare, said: “People who are vulnerable to acquiring HIV, especially those in Black and Latinx communities who are disproportionately impacted in the US, may want options beyond daily oral pills. That’s why ViiV Healthcare is proud that Apretude was studied in one of the most diverse and comprehensive HIV prevention trial programs to date, which also included some of the largest numbers of transgender women and Black men who have sex with men ever enrolled in an HIV prevention trial. With Apretude, people can reduce the risk of acquiring HIV with as few as six injections a year. Today’s approval is the latest example of ViiV Healthcare’s commitment to developing long-acting medicines that offer consumers a different choice.”
US FDA approval is based on the results from two international phase IIb/III multicenter, randomised, double-blind, active-controlled trials, HPTN 083 and HPTN 084, which evaluated the safety and efficacy of cabotegravir long-acting for PrEP in HIV-negative men who have sex with men, transgender women, and cisgender women, who were at increased risk of sexually acquiring HIV. In these trials, which included more than 7,700 participants across 13 countries combined, the blinded, randomised portions of both trials were stopped early by an independent Data Safety Monitoring Board after cabotegravir long-acting for PrEP was shown to be superior to daily oral emtricitabine/tenofovir disoproxil fumarate (TDF/FTC) tablets in preventing the acquisition of HIV in study participants. Clinical trial participants who received cabotegravir long-acting for PrEP experienced a 69% lower incidence of HIV compared to FTC/TDF tablets in HPTN 083 and a 90% lower incidence of HIV compared to FTC/TDF tablets in HPTN 084.
The most common adverse reactions (all grades) observed in at least 1% of clinical trial participants receiving cabotegravir long-acting for PrEP were injection site reactions, diarrhoea, headache, pyrexia, fatigue, sleep disorders, nausea, dizziness, flatulence, abdominal pain, vomiting, myalgia, rash, decreased appetite, somnolence, back pain, and upper respiratory tract infection. Adverse events led to discontinuation in 6% of participants in HPTN 083 and 1% of participants in HPTN 084.
In HPTN 083, participants in the US were inclusive of the Black/African American and Latinx communities of men and transgender women who have sex with men, who are disproportionately affected by the HIV epidemic and comprise the greatest percentage of new HIV diagnoses. In HPTN 084, all participants were cisgender women from sub-Saharan Africa. Women in this region bear a disproportionate burden of the HIV epidemic and may be twice as likely to acquire HIV as their male counterparts.
Richard Elion, MD, Director of Research at Washington Health Institute, said: “We have the tools to end the HIV epidemic through the implementation of effective antiretroviral treatment and HIV prevention. PrEP has played a vital role in protecting people from acquiring HIV. With the availability of cabotegravir long-acting for PrEP as an injection every two months to prevent HIV, people now have an important new option besides daily medication. This long-acting medication offers more options for prevention, and now providers and patients will be empowered by choices and the ability to choose the approach that is optimal for each individual.”
HIV continues to be a global public health crisis, with an estimated 38 million people living with HIV worldwide and 1.7 million new cases annually. PrEP represents an effective tool to reduce new cases of HIV, which in addition to successful HIV antiretroviral treatment, will help efforts to end the HIV epidemic. However, fewer than 25% of the people who could benefit from PrEP in the US are currently taking it. Despite the wide availability of daily oral PrEP, it can be limited by inconsistent adherence as well as structural and cultural barriers that lead to underutilisation in key populations.
https://www.drugs.com/apretude.html
https://www.drugs.com/newdrugs/fda-approves-apretude-cabotegravir-extended-release-injectable-suspension-hiv-pre-exposure-5754.html
Saturday, May 18, 2019
FDA Approves Dovato (dolutegravir/lamivudine) for HIV-1 Infection

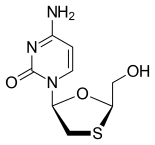
Pedro Cahn, principal investigator for the GEMINI study program said: “People are now living longer with HIV and will spend a lifetime taking drugs to suppress their virus. The approval of the fixed dose combination of dolutegravir and lamivudine, a complete, single-tablet, two-drug regimen, marks a pivotal moment in the treatment of HIV-1. Treatment-naïve people living with the virus have a powerful option that delivers non-inferior efficacy to a dolutegravir-based three-drug regimen, allowing them to take fewer ARVs and get and remain suppressed.”
About Dovato (dolutegravir/lamivudine)
https://en.wikipedia.org/wiki/Lamivudine
Saturday, November 17, 2018
FDA Approves Merck’s Pifeltro (doravirine) for the Treatment of HIV-1 in Appropriate Patients

Friday, November 16, 2018
FDA Approves Merck’s Pifeltro (doravirine) for the Treatment of HIV-1 in Appropriate Patients

Thursday, November 15, 2018
FDA Approves Merck’s Delstrigo (doravirine/lamivudine/tenofovir disoproxil fumarate) for the Treatment of HIV-1 in Appropriate Patients
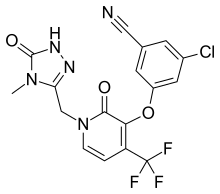

doravirine Lamivudine Tenofovir
Data Supporting the Approval of Delstrigo (doravirine 100 mg/3TC 300 mg/TDF 300 mg)
Wednesday, November 14, 2018
FDA Approves Merck’s Delstrigo (doravirine/lamivudine/tenofovir disoproxil fumarate) for the Treatment of HIV-1 in Appropriate Patients

The combination of Imbruvica and rituximab provides health care professionals with a new treatment option for patients living with this serious blood cancer,” said Dr. Lia Palomba, hematologist-oncologist at Memorial Sloan-Kettering Cancer Center, New York, and iNNOVATE study investigator. “Before Imbruvica, there were no FDA-approved treatment options for patients with Waldenström’s macroglobulinemia, a disease first acknowledged nearly 75 years ago. Today, Imbruvica continues to provide an important therapeutic approach in the treatment of this complex disease.”
“Results from iNNOVATE showed significant improvement in progression-free survival at 30 months and demonstrated the superiority of Imbruvica plus rituximab over rituximab monotherapy in Waldenström's macroglobulinemia,” said Meletios A. Dimopoulos, M.D., Professor and Chairman of the Department of Clinical Therapeutics, National and Kapodistrian University of Athens School of Medicine, Athens, Greece, and iNNOVATE lead study investigator. “Based on these results, Imbruvica in combination with rituximab may be considered as a first- and second-line option for appropriate people diagnosed and living with WM.”
“The clinical data generated for Imbruvica plus rituximab in the treatment of Waldenström’s macroglobulinemia offers physicians evidence to consider this combination regimen for newly-diagnosed patients. Today’s approval represents an important milestone for people living with this rare and incurable blood cancer who have limited FDA-approved treatment options,” said Andree Amelsberg, M.D., Vice President of Oncology Medical Affairs at Janssen Scientific Affairs, LLC. “We remain dedicated to a comprehensive clinical development program to explore the full potential of Imbruvica, including in combination with other therapies.”
Thursday, July 5, 2018
Single-tablet HIV treatment shows better outcomes over multi-tablet regimen

"There were not differences in adherence as we could measure it via pharmacy refill dates, which suggests that maybe the single-tablet regimens are more efficacious," he said. "It could also be that the persons who got the multi-tablet regimens had more barriers to care and that is why they did more poorly." He says more studies will be needed to help tease out the differences in the types of medications being used versus the effect of pill burden.
Wednesday, May 23, 2018
Mylan Introduces Symfi (efavirenz, lamivudine and tenofovir disoproxil fumarate) Triple Combo Once-Daily HIV Treatment in the U.S.


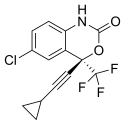
"As the largest supplier of antiretrovirals by volume in the world, Mylan has a longstanding commitment to expanding affordable access to treatments for people living with HIV," said Mylan CEO Heather Bresch. "As we continue to grow our U.S. portfolio of ARV products, now including Symfi Lo™, Symfi™, and Cimduo™, we are providing access to patients and empowering them to choose the lower-cost ARV treatment option that is right for them."
"Mylan has been on the forefront of bringing innovative delivery and dosage forms of ARVs to millions of patients in the developing world," said Mylan President Rajiv Malik. "We've already extended our reach to people in the U.S. living with HIV with the introduction of Symfi Lo™ and Cimduo™. Adding Symfi™ to our portfolio further strengthens our commitment to investing in developing and manufacturing these important products."
Monday, April 16, 2018
Antiviral drug not beneficial for reducing mother-to-child transmission of hepatitis B when added to existing preventatives
“We observed no treatment-related safety concerns for the mothers or infants and no significant differences in infant growth,” said the study’s lead author Gonzague Jourdain, M.D., Ph.D., of Thailand’s Chiang Mai University, the Harvard T.H. Chan School of Public Health and France’s IRD (Institut de recherche pour le développement). “These safety data also are relevant for pregnant women receiving TDF as part of HIV treatment or HIV pre-exposure prophylaxis.”
Thursday, July 7, 2016
TDF, entecavir duo 'highly effective' for difficult-to-treat chronic HBV

Thursday, May 26, 2016
FDA Approves Odefsey (emtricitabine, rilpivirine and tenofovir alafenamide) for the Treatment of HIV-1 Infection
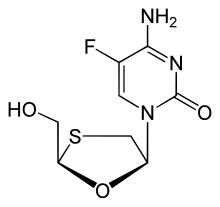 Emtricitabine
Emtricitabine  Rilpivirine
Rilpivirine 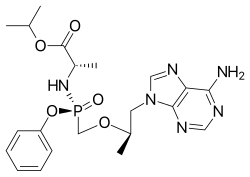 Tenofovir alafenamide
Tenofovir alafenamideTuesday, June 11, 2013
Bristol-Myers Squibb Receives US FDA sNDA Approval for Use of SUSTIVA® (efavirenz) in HIV-1 Infected Pediatric Patients | BMS Newsroom
Monday, May 21, 2012
Truvada deemed safe & effective in HIV infection risk reduction
According to the Food and Drug Administration Truvada - a combination of Gilead's HIV drugs Emtriva (also known as emtricitabine see above structure), and Viread (or tenofovir see below left structure), which is already being used by patients with the human immunodeficiency virus, is well (left structure is that of Tenofovir disoproxil fumarate) tolerated overall by uninfected people and may prevent infection in high-risk individuals when used in combination with other strategies. The FDA acknowledged a strong correlation between the drug's efficacy at reducing HIV infection and the willingness of those taking it to adhere to the treatment.





