Friday, January 2, 2026
FDA Approves Inlexzo (gemcitabine intravesical system) for the Treatment of Non-Muscle Invasive Bladder Cancer
Thursday, July 22, 2021
Progress towards new treatments for tuberculosis
Tuberculosis still represents an enormous global disease burden and is one of the top 10 causes of death worldwide.
Led by WEHI's Dr. Michael Stutz and Professor Marc Pellegrini and published in Immunity, the study uncovered how cells infected with tuberculosis bacteria can die, and that using new medicines to enhance particular forms of cell death decreased the severity of the disease in a preclinical model.
Fighting antibiotic resistance
Tuberculosis is caused by bacteria that infect the lungs, spreading from person to person through the air. A challenge in the fight against tuberculosis is that the bacteria that cause the disease have developed resistance to most antibiotic treatments, leading to a need for new treatment approaches.
Tuberculosis bacteria grow within immune cells in the lungs. One of the ways that cells protect against these 'intracellular' pathogens is to undergo a form of cell death called apoptosis—destroying the cell as well as the microbes within it.
Using preclinical models, researchers sequentially deleted key apoptosis effectors, to demonstrate their roles in controlling tuberculosis infections. This demonstrated that a proportion of tuberculosis-infected cells could die by apoptosis—opening up new opportunities for controlling the disease.
Using host-directed therapies to reduce disease burden
Dr. Stutz said researchers then tested new drugs that force cells to die. This revealed that a drug-like compound that inhibits cell death-regulatory proteins called IAPs could promote death of the infected cells.
"When we treated our infection models with this compound, we were able to significantly reduce the amount of tuberculosis disease," he said.
"The longer the treatment was used, the greater the reduction of disease."
The research team was able to replicate these results using various different IAP inhibitors.
"Excitingly, many of these compounds are already in clinical trials for other types of diseases and have proven to be safe and well-tolerated by patients," Dr. Stutz said.
"We predict that if these compounds were progressed for treating tuberculosis, they would be most effective if used alongside existing antibiotic treatments."
Opening the door to new treatment methods
Professor Marc Pellegrini said until now, antibiotics were the only treatment for tuberculosis, which were limited in their application due to increasing antibiotic resistance.
"Unlike antibiotics, which directly kill bacteria, IAP inhibitors kill the cells that the tuberculosis bacteria need to survive," he said.
"The beauty of using a host-directed therapy is that it doesn't directly target the microbe, it targets a host process. By targeting the host rather than the microbe, the chances of resistance developing are incredibly low."
The team hope the research will lead to better treatments for tuberculosis.
"This research increases our understanding of the types of immune responses that are beneficial to us, and this is an important step towards new treatments for tuberculosis, very few of which have been developed in the last 40 years," Dr. Stutz said.
"We have demonstrated that host-directed therapies are viable for infections such as tuberculosis, which is particularly important in the era of extensive antibiotic resistance."
https://www.sciencedirect.com/science/article/abs/pii/S1074761321002533?via%3Dihub
Tuesday, April 6, 2021
Newly discovered antibiotics kill bacteria differently
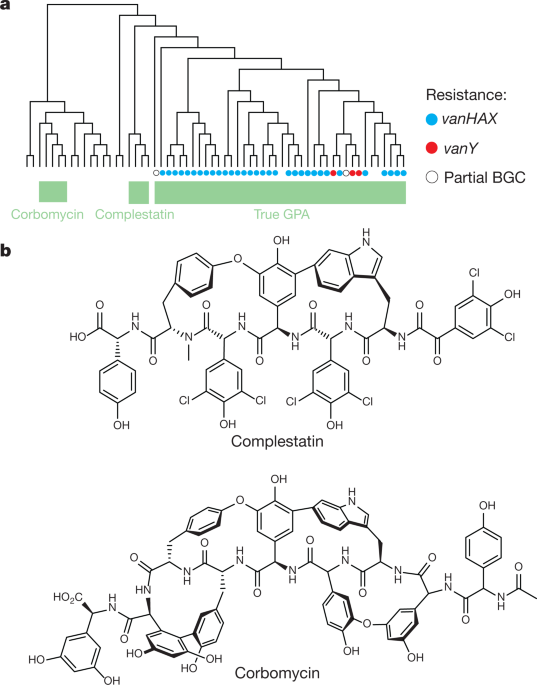
'Holy grail' of antibiotics
Unique bacteria killer
Fight against antibiotic resistance
Tuesday, June 30, 2020
New treatment kills off infection that can be deadly to cystic fibrosis patients
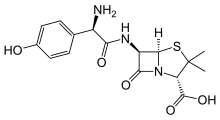
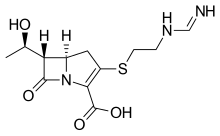
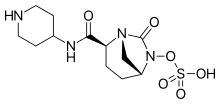 Relebactam
Relebactam"This shows our drugs, when used in combination, are widely effective and could therefore make a huge difference to people whose treatment options are currently limited.
"Because amoxicillin is already widely available and imipenem-relebactam has just been approved for use by the Food and Drug Administration (FDA) in the US, these drugs are already available to clinicians. We therefore hope to start treating patients as soon as possible. "
"Mycobacterium abscessus also known as NTM, is the most feared infection a person with cystic fibrosis can develop. Taking drugs to treat NTM can add to an already significant regime of daily treatments and take up to a year to clear infections. We look forward to a time when effective, short courses of treatment are available to treat NTM."
https://en.wikipedia.org/wiki/Amoxicillin
https://en.wikipedia.org/wiki/Imipenem https://en.wikipedia.org/wiki/Relebactam
https://medicalxpress.com/news/2019-10-fda-drug-common-cystic-fibrosis.html
Thursday, February 27, 2020
FDA Approves Pretomanid for Highly Drug-Resistant Forms of Tuberculosis
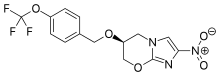
“FDA approval of this treatment represents a victory for the people suffering from these highly drug-resistant forms of the world’s deadliest infectious disease,” said Mel Spigelman, MD, president and CEO of TB Alliance. “The associated novel regimen will hopefully provide a shorter, more easily manageable and highly efficacious treatment for those in need.”
“Until very recently, people infected with highly drug-resistant TB had poor treatment options and a poor prognosis,” said Dr. Francesca Conradie, principal investigator of the Nix-TB trial. “This new regimen provides hope with 9 out of 10 patients achieving culture negative status at 6 months post-treatment with this short, all-oral regimen."
Thursday, January 23, 2020
FDA Approves Rinvoq (upadacitinib), an Oral JAK Inhibitor for the Treatment of Moderate to Severe Rheumatoid Arthritis

"Despite the availability of multiple treatment options with varying mechanisms of action, many patients still do not achieve clinical remission or low disease activity—the primary treatment goals for rheumatoid arthritis," said Roy M. Fleischmann, M.D., primary investigator for SELECT-COMPARE and clinical professor at the University of Texas Southwestern Medical Center at Dallas. "With this FDA approval, Rinvoq has the potential to help additional people living with RA achieve remission who have not yet reached this goal."
- In SELECT-EARLY, 52 percent of MTX-naïve patients treated with Rinvoq 15 mg achieved ACR50 vs 28 percent treated with MTX at week 121
- In SELECT-MONOTHERAPY, 68 percent of MTX-IR patients treated with Rinvoq 15 mg achieved ACR20 vs 41 percent treated with continued MTX at week 141
- In SELECT-COMPARE, 71 percent of MTX-IR patients treated with Rinvoq 15 mg plus MTX achieved ACR20 vs 36 percent treated with placebo plus MTX at week 121
- In SELECT-NEXT, 64 percent of csDMARD-IR patients treated with Rinvoq 15 mg plus csDMARDs achieved ACR20 vs 36 percent treated with placebo plus csDMARDs at week 121
- In SELECT-BEYOND, 65 percent of biologic-IR patients treated with Rinvoq 15 mg plus csDMARDs achieved ACR20 vs 28 percent treated with placebo plus csDMARDs at week 121
"The discovery and development of Rinvoq is indicative of AbbVie's long-standing commitment to advancing the science for people living with immune-mediated conditions," said Michael Severino, M.D., vice chairman and president, AbbVie. "Today's FDA approval marks an important milestone in our pursuit to deliver innovative medicines that advance care for people living with rheumatoid arthritis."
Safety
Ease of Use and Access
"Rheumatoid arthritis can have a debilitating impact on the lives of those with the chronic disease, including making it difficult to perform everyday tasks," said Cindy McDaniel, senior vice president, consumer health, Arthritis Foundation. "The Arthritis Foundation is committed to recognizing innovation that can help patients living with rheumatoid arthritis and we are proud to recognize AbbVie with our Ease of Use Commendation for the packaging design of Rinvoq."
Tuesday, May 7, 2019
TB Medicine Pretomanid Enters Regulatory Review Process in the United States

About Pretomanid and the BPaL Regimen
Wednesday, September 5, 2018
FDA Approves Olumiant (baricitinib) 2 mg Tablets for the Treatment of Adults with Moderately-to-Severely Active Rheumatoid Arthritis

"We are pleased to provide RA patients in the U.S. an effective treatment option with Olumiant, as people with RA who have had an inadequate response to TNF inhibitors are generally considered to be some of the most difficult to treat RA patients," said Christi Shaw, president, Lilly Bio-Medicines.
"Despite the advancements we've seen in the RA treatment landscape over the past several decades, many patients are still failing to achieve their disease management goals," said Seth Ginsberg, co-founder and president of CreakyJoints and the Global Healthy Living Foundation. "As it's important for RA patients to have multiple treatment options available to best suit their disease characteristics and experiences, the approval of Olumiant is very encouraging for our community."
"In my clinical practice, I continue to see patients who experience debilitating symptoms and who are waiting for a medicine that may be right for them," said Elizabeth L. Perkins, M.D., Rheumatology Care Center, Birmingham, Alabama. "Olumiant is an important option for rheumatologists to help address these patients' unmet needs."
"RA patients continue to experience unique challenges accessing the treatments prescribed by their healthcare providers. Therefore, we are determined to continue our work with stakeholders to demonstrate value across the healthcare system so providers have greater choice in prescribing treatments to fit individual patient needs," said Shaw.
Wednesday, August 1, 2018
Antimicrobial peptides are promising alternative for combatting antimicrobial resistance
“Nanoparticles can overcome the major obstacle to peptide-based therapies that promise much in the fight against antimicrobial-resistant infections. For example, in the project we identified highly effective AMPs to combat tuberculosis. This is so promising that we are now seeking collaborators and funding for further development and to move towards eventual clinical testing.”
Fast, controlled delivery
And the benefits go beyond the ‘magic bullet’ effect of the nanoparticles, says Ringstad. “Most conventional antibiotics are delivered through pills or injections, and if they underperform then more are prescribed. We have focused on treating skin and lung infection locally, thereby reducing exposure and making treatment easier for the patient. Local delivery strategies using nanoparticles can be more cost-effective, as they use less of the active ingredient, and have fewer side effects for the same reason.”
“One important result concerned the effect of nanoparticles on biofilms,” explains Ringstad. “Biofilms are aggregations of infectious bacteria which protect the infected area against antibiotics and other therapies – they are common in many types of infection and are difficult to penetrate. We found that when nanoparticles are loaded with AMPs then the degradation of the biofilm was significantly improved. This ability to successfully attack biofilms is a very significant result for treating conditions such as cystic fibrosis and burn wound infections.”
Ringstad emphasizes the importance of FORMAMP results. “The nanoparticle delivery mechanism is not limited to treating infections – it could be used in a broad range of therapies. With further research, nanotherapeutics could possibly deliver more effective treatments with fewer side effects and at a lower cost for a wide range of conditions”.
Wednesday, January 10, 2018
New small-molecule drug restores brain function, memory in mouse model of Alzheimer's disease
An international team of researchers has shown that a new small-molecule drug can restore brain function and memory in a mouse model of Alzheimer's disease. The drug works by stopping toxic ion flow in the brain that is known to trigger nerve cell death. Scientists envision that this drug could be used to treat Alzheimer's and other neurodegenerative diseases such as Parkinson's and ALS.
"This is the first drug molecule that can regulate memory loss by directly blocking ions from leaking through nerve cell membranes," said Ratnesh Lal, a professor of bioengineering at the University of California San Diego and co-senior author of the study.
While collaborators in Germany will be pursuing clinical studies in human patients with neurodegenerative diseases, Lal and his research group at the UC San Diego Jacobs School of Engineering are particularly interested in testing anle138b on a variety of other diseases that are linked to toxic ion flow caused by amyloid proteins, including diabetes, tuberculosis and certain types of cancer. Lal's group has performed extensive research on amyloid ion channels and their roles in these diseases. "Blocking the ion leakiness of amyloid channels using anle138b could be an effective therapy for various diseases," Lal said.


