Epizyme, Inc. (Nasdaq: EPZM), a biopharmaceutical company developing novel epigenetic therapies, announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval of Tazverik (tazemetostat) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection, based on overall response rate and duration of response in a Phase 2 clinical trial.
Despite industry advancements, there are limited therapeutic options for treating patients with epithelioid sarcoma who struggle with high rates of recurrence and toxicities associated with currently used therapies,” said Gary K. Schwartz, M.D., chief of hematology and oncology at Columbia University and NewYork-Presbyterian Hospital, deputy director of the Herbert Irving Comprehensive Cancer Center, professor of oncology at Columbia University Vagelos College of Physicians and Surgeons and an investigator in Epizyme’s Phase 2 trial. “The Tazverik data from the ES cohort in Epizyme’s Phase 2 trial support its potential to provide clinically meaningful and durable responses, and tolerability for ES patients. This approval of Tazverik represents an important advancement in the treatment of patients with ES.”
“Today’s accelerated approval of Tazverik is a landmark event for people with ES and represents our dedication to our mission of rewriting treatment for people with cancer and other serious diseases,” said Robert Bazemore, president and chief executive officer of Epizyme. “Tazverik is now the first and only FDA-approved EZH2 inhibitor, and the first and only FDA-approved treatment specifically indicated for ES patients. Our commercial launch plans are underway, and we expect to make Tazverik available to ES patients and treating physicians across the U.S. within 10 business days.”
“For people with epithelioid sarcoma, an aggressive life threatening cancer that affects young adults, having new treatment options can offer much needed hope,” added Denise Reinke, MS, NP, MBA, president and chief executive officer of the Sarcoma Alliance for Research through Collaboration (SARC) and co-founder of the Sarcoma Coalition.
Dr. Shefali Agarwal, chief medical officer, commented, “Discovering, developing and obtaining FDA approval for Tazverik, with its novel mechanism of action, is the result of years of work and commitment by many people, including the patients, caregivers and physicians who have participated in our clinical trials, along with the talented team at Epizyme. We are tremendously proud of this important milestone and look forward to further advancing clinical development of tazemetostat for multiple types of cancers.”
Continued approval for this indication is contingent upon verification and description of clinical benefit in a confirmatory trial. The company’s ongoing, global, randomized, controlled confirmatory trial assessing the combination of Tazverik plus doxorubicin compared with doxorubicin plus placebo as a front-line treatment for ES is underway.
In addition, Epizyme will conduct certain post-marketing activities, including clinical pharmacology evaluations to assess the effect of Tazverik on liver function and the effect of CYP3A inhibitors and inducers on Tazverik to inform aspects of the prescribing information. The company will also expand enrollment in Cohort 6 of its Phase 2 study, which has enrolled 44 patients to date, for a total of at least 60 epithelioid sarcoma patients. This expansion is intended to provide more patient experience for potential future inclusion in the label.
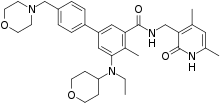
https://en.wikipedia.org/wiki/Tazemetostat
FDA Approves Tazverik (tazemetostat) for the Treatment of Patients with Epithelioid Sarcoma
