Jazz Pharmaceuticals plc announced that the U.S. Food and Drug Administration (FDA) approved Sunosi (solriamfetol) to improve wakefulness in adult patients with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea (OSA). Once-daily Sunosi is approved with doses of 75 mg and 150 mg for patients with narcolepsy and doses of 37.5 mg, 75 mg, and 150 mg for patients with OSA. Sunosi is the first dual-acting dopamine and norepinephrine reuptake inhibitor (DNRI) approved to treat excessive daytime sleepiness in adults living with narcolepsy or OSA.
Sunosi is expected to be commercially available in the U.S. following the final scheduling decision by the U.S. Drug Enforcement Administration (DEA), which is typically within 90 days of FDA approval.
"Excessive daytime sleepiness can negatively impact the daily lives of people living with narcolepsy or obstructive sleep apnea at work, at home or in daily activities. With this approval, a new, daytime medicine that can provide sustained wakefulness throughout the day will be available for patients," said Bruce Cozadd, chairman and chief executive officer of Jazz Pharmaceuticals. "The FDA approval of Sunosi also represents an important milestone for Jazz as we continue to offer new treatment options that address unmet needs for people living with chronic, and often debilitating, sleep disorders."
At Week 12, 150 mg of Sunosi for narcolepsy patients and all doses for OSA patients demonstrated improvements in wakefulness compared to placebo as assessed in test sessions 1 (approximately one hour post-dose) through 5 (approximately nine hours post-dose) of the maintenance of wakefulness test (MWT).
The FDA's approval of Sunosi is based on data from the Treatment of Obstructive sleep apnea and Narcolepsy Excessive Sleepiness (TONES) Phase 3 clinical program, which included four randomized placebo-controlled studies that demonstrated the superiority of Sunosi relative to placebo. The most common adverse reactions (incidence ≥5% and higher than placebo) reported in both the narcolepsy and OSA study populations were headache, nausea, decreased appetite, and anxiety. Sunosi was evaluated in more than 900 adults with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea and was shown to maintain its effect relative to placebo after six months of use.
"We're excited about this new therapeutic option for patients, and we are pleased with the information included in the Sunosi label as we believe it will give physicians the information needed to appropriately manage the vast majority of obstructive sleep apnea and narcolepsy patients with excessive daytime sleepiness," said Daniel Swisher, president and chief operating officer of Jazz Pharmaceuticals.
In 12 week clinical studies, approximately 68-74% of people taking Sunosi at the 75 mg dose and 78-90% of people taking Sunosi at the 150 mg dose reported improvement in their overall clinical condition, as assessed by the Patient Global Impression of Change (PGIc) scale.
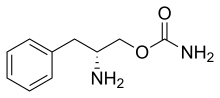
Although the exact mechanism of action is unknown, the effects of Sunosi are thought to be mediated through its activity as a DNRI.
"Sunosi is an effective treatment option with a novel mechanism of action as a dual-acting dopamine and norepinephrine reuptake inhibitor," said Richard K. Bogan, MD, FCCP, FAASM, Associate Clinical Professor at the University of South Carolina School of Medicine and Chief Medical Officer at SleepMed in Columbia, SC. "Excessive daytime sleepiness is the most common symptom for people with narcolepsy and a major complaint of people with obstructive sleep apnea. In some people with obstructive sleep apnea, excessive daytime sleepiness may persist despite using CPAP."
Sunosi is not indicated to treat the underlying airway obstruction in OSA. Ensure that the underlying airway obstruction is treated (e.g., with continuous positive airway pressure (CPAP)) for at least one month prior to initiating Sunosi for excessive daytime sleepiness in OSA. Modalities to treat the underlying airway obstruction should be continued during treatment with Sunosi. Sunosi is not a substitute for these modalities.
About Sunosi (solriamfetol)
Sunosi is a dual-acting dopamine and norepinephrine reuptake inhibitor (DNRI) indicated to improve wakefulness in adults living with excessive daytime sleepiness due to narcolepsy or obstructive sleep apnea (OSA). In 2014, Jazz Pharmaceuticals acquired a license to develop and commercialize Sunosi from Aerial Biopharma. Jazz Pharmaceuticals has worldwide development, manufacturing, and commercialization rights to Sunosi, excluding certain jurisdictions in Asia. SK Biopharmaceuticals, the discoverer of the compound, maintains rights in 12 Asian markets, including Korea, China and Japan. Sunosi has orphan drug designation for narcolepsy in the United States.
Important Safety Information
Sunosi can be a target for people who abuse prescription medicines or street drugs. Keep Sunosi in a safe place to protect it from theft. Never give your Sunosi to anyone else, because it may cause death or harm them. Selling or giving away Sunosi may harm others and is against the law. Tell your doctor if you have ever abused or been dependent on alcohol, prescription medicines or street drugs.
Do not take Sunosi if you are taking or have stopped taking within the past 14 days a medicine used to treat depression called a monoamine oxidase inhibitor (MAOI).
Before taking Sunosi, tell your doctor about all of your medical conditions, including if you:
- have heart problems, high blood pressure, kidney problems, diabetes, or high cholesterol
- have had a heart attack or a stroke
- have kidney problems or diabetes
- have a history of mental health problems, (including psychosis and bipolar disorders), or of drug or alcohol abuse or addiction
- are pregnant or planning to become pregnant. It is not known if Sunosi will harm your unborn baby.
- Pregnancy Registry: There is a pregnancy registry for women who take Sunosi during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your doctor about how you can take part in this registry.
- are breastfeeding or plan to breastfeed. It is not known if Sunosi passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take Sunosi.
Especially tell your healthcare provider if you take a medicine used to treat depression called a monoamine oxidase inhibitor (MAOI).
Ref : https://en.wikipedia.org/wiki/Solriamfetol
