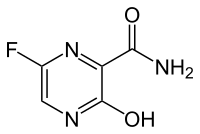
We know that, Favipiravir, also known as T-705 or Avigan, is an experimental antiviral drug being developed by Toyama Chemical of Japan with activity against many RNA viruses. Like some other experimental antiviral drugs (T-1105 and T-1106), it is a pyrazinecarboxamide derivative. Favipiravir is active against influenza viruses, West Nile virus, yellow fever virus, foot-and-mouth disease virus as well as other flaviviruses, arenaviruses, bunyaviruses and alphaviruses.[1Activity against enteroviruses and Rift Valley fever virus has also been demonstrated.
The mechanism of its actions is thought to be related to the selective inhibition of viral RNA-dependent RNA polymerase.[4] Favipiravir does not inhibit RNA or DNA synthesis in mammalian cells and is not toxic to them.[1]
In 2014, favipiravir was approved in Japan for stockpiling against influenza pandemics
Favipiravir, an investigational antiviral drug currently being tested in West Africa as a treatment for Ebola virus disease, effectively treated Lassa virus infection in guinea pigs, according to a new study from National Institutes of Health (NIH) scientists and colleagues. Lassa fever is endemic to West Africa and affects about 300,000 people annually, killing roughly 5,000. In some parts of Sierra Leone and Liberia, it is believed nearly 15 percent of people admitted to hospitals have Lassa fever, according to the Centers for Disease Control and Prevention. No vaccine or licensed treatment exists for Lassa fever, although ribavirin, licensed for hepatitis C treatment, has been used with limited success. In the new study, published Oct. 12, 2015, in Scientific Reports, favipiravir not only effectively treated guinea pigs infected with Lassa virus, it also worked better than ribavirin.
Two days after infecting groups of guinea pigs with a lethal dose of Lassa virus, the scientists treated the rodents daily for two weeks with either ribavirin, low doses of favipiravir, or high doses of favipiravir. They also evaluated the effect of high-dose favipiravir in the rodents that began treatment five, seven or nine days after infection. All of the animals that received high-dose favipiravir were completely protected from lethal infection; animals treated seven or nine days after infection had begun showing signs of disease, but their conditions quickly improved when treatment began. Those animals in the low-dose favipiravir group showed mild to moderate signs of disease, but those symptoms resolved after about one week of treatment. The animals treated with ribavirin appeared normal during the treatment phase but developed severe disease shortly after treatment ended.
