Thursday, August 29, 2024
Drug Used to Treat Rheumatoid Arthritis May Also Help Prevent It
Friday, March 22, 2019
AbbVie Announces New Drug Application Accepted for Priority Review by FDA for Upadacitinib for Treatment of Moderate to Severe Rheumatoid Arthritis

About the SELECT Study Program
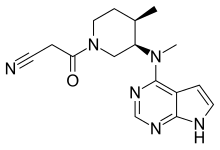
About Upadacitinib
Monday, August 31, 2015
Chemical compound shows promise in treating rheumatoid arthritis
is a leading scientific journal that covers all aspects of pharmacology, a field that investigates the effects of drugs on biological systems and vice versa.
College of Letters and Science.
them, Quinn said. Some people respond at first, but not forever.
works against rheumatoid arthritis. They explained their findings in the JPET paper.
Chemical compound shows promise in treating rheumatoid arthritis:
Thursday, January 23, 2020
FDA Approves Rinvoq (upadacitinib), an Oral JAK Inhibitor for the Treatment of Moderate to Severe Rheumatoid Arthritis

"Despite the availability of multiple treatment options with varying mechanisms of action, many patients still do not achieve clinical remission or low disease activity—the primary treatment goals for rheumatoid arthritis," said Roy M. Fleischmann, M.D., primary investigator for SELECT-COMPARE and clinical professor at the University of Texas Southwestern Medical Center at Dallas. "With this FDA approval, Rinvoq has the potential to help additional people living with RA achieve remission who have not yet reached this goal."
- In SELECT-EARLY, 52 percent of MTX-naïve patients treated with Rinvoq 15 mg achieved ACR50 vs 28 percent treated with MTX at week 121
- In SELECT-MONOTHERAPY, 68 percent of MTX-IR patients treated with Rinvoq 15 mg achieved ACR20 vs 41 percent treated with continued MTX at week 141
- In SELECT-COMPARE, 71 percent of MTX-IR patients treated with Rinvoq 15 mg plus MTX achieved ACR20 vs 36 percent treated with placebo plus MTX at week 121
- In SELECT-NEXT, 64 percent of csDMARD-IR patients treated with Rinvoq 15 mg plus csDMARDs achieved ACR20 vs 36 percent treated with placebo plus csDMARDs at week 121
- In SELECT-BEYOND, 65 percent of biologic-IR patients treated with Rinvoq 15 mg plus csDMARDs achieved ACR20 vs 28 percent treated with placebo plus csDMARDs at week 121
"The discovery and development of Rinvoq is indicative of AbbVie's long-standing commitment to advancing the science for people living with immune-mediated conditions," said Michael Severino, M.D., vice chairman and president, AbbVie. "Today's FDA approval marks an important milestone in our pursuit to deliver innovative medicines that advance care for people living with rheumatoid arthritis."
Safety
Ease of Use and Access
"Rheumatoid arthritis can have a debilitating impact on the lives of those with the chronic disease, including making it difficult to perform everyday tasks," said Cindy McDaniel, senior vice president, consumer health, Arthritis Foundation. "The Arthritis Foundation is committed to recognizing innovation that can help patients living with rheumatoid arthritis and we are proud to recognize AbbVie with our Ease of Use Commendation for the packaging design of Rinvoq."
Thursday, February 20, 2020
FDA Approves RediTrex (methotrexate) for Rheumatoid Arthritis, Juvenile Idiopathic Arthritis, and Psoriasis

Monday, February 12, 2018
Pfizer Announces FDA Approval of Xeljanz (tofacitinib) and Xeljanz XR for the Treatment of Active Psoriatic Arthritis
“Psoriatic arthritis is a complex and progressive disease with an unpredictable course,” said Angela Hwang, Global President, Inflammation and Immunology, Pfizer. “The approval of Xeljanz is an important step forward for patients seeking new treatments and is a testament to Pfizer’s unwavering commitment to advancing patient care.”
“As a practicing rheumatologist, I’ve seen the significant physical impact psoriatic arthritis has on people living with the disease, and many patients are looking for additional therapeutic options,” said Philip Mease, M.D., Swedish Medical Center, University of Washington and study investigator. “I’m pleased that Xeljanz is now available for use in the treatment of this chronic condition.”
“Psoriatic arthritis is a serious and debilitating chronic illness that should be diagnosed and treated early,” said Randy Beranek, president and CEO, National Psoriasis Foundation. “As an organization that advocates for people living with psoriatic arthritis, we welcome the availability of new therapies for treating this disease.”
Sunday, April 19, 2009
Simponi the first biologic therapy to be approved for rheumatologic diseases !
Humira (brand name is an abbreviation of "Human Monoclonal Antibody in Rheumatoid Arthritis") is marketed in both preloaded 0.8 ml syringes and also in preloaded pen devices (called Humira Pen), both injected subcutaneously, typically by the patient at home. It cannot be administered orally, because the digestive system would destroy the drug. But its now the turn of Golimumab, a new fully human monoclonal antibody. Being a fully human MAb directed against TNF, Golimumab resembles Adalimumab (Humira, Abbott), which was the first such product to reach the market. Now the Canadian government has approved Golimumab along with ‘methotrexate’ for the treatment of three forms of Rheumatiod arthritis (Rheumatoid Arthritis, Ankylosing Spondylitis & Psoriatic Arthritis) and more over making this treatment the first biologic therapy to be approved.
With this approval in Canada, Simponi (Golimumb), in combination with methotrexate (MTX), is indicated for reducing the signs and symptoms in adult patients with moderately to severely active RA; reducing signs and symptoms in adult patients with moderately to severely active PsA, alone or in combination with MTX; and reducing signs and symptoms in adult patients with active AS who have had an inadequate response to conventional therapies. More...
Wednesday, September 5, 2018
FDA Approves Olumiant (baricitinib) 2 mg Tablets for the Treatment of Adults with Moderately-to-Severely Active Rheumatoid Arthritis

"We are pleased to provide RA patients in the U.S. an effective treatment option with Olumiant, as people with RA who have had an inadequate response to TNF inhibitors are generally considered to be some of the most difficult to treat RA patients," said Christi Shaw, president, Lilly Bio-Medicines.
"Despite the advancements we've seen in the RA treatment landscape over the past several decades, many patients are still failing to achieve their disease management goals," said Seth Ginsberg, co-founder and president of CreakyJoints and the Global Healthy Living Foundation. "As it's important for RA patients to have multiple treatment options available to best suit their disease characteristics and experiences, the approval of Olumiant is very encouraging for our community."
"In my clinical practice, I continue to see patients who experience debilitating symptoms and who are waiting for a medicine that may be right for them," said Elizabeth L. Perkins, M.D., Rheumatology Care Center, Birmingham, Alabama. "Olumiant is an important option for rheumatologists to help address these patients' unmet needs."
"RA patients continue to experience unique challenges accessing the treatments prescribed by their healthcare providers. Therefore, we are determined to continue our work with stakeholders to demonstrate value across the healthcare system so providers have greater choice in prescribing treatments to fit individual patient needs," said Shaw.
Monday, December 14, 2009
Methotrexate & Ocrelizumab combination a new hope for RA patients....
About monoclonal antibodies :
 Many monoclonal antibodies like infliximab, etanercept and adalimumab were tried for the rheumatoid arthritis now its interseting to note that Genentech and Biogen Idec reported positive outcome from ocrelizumab ( humanized anti-CD20) -MTX (Methotrexate - see the structure : this drug is a part of DMARD treatment meant for RA patients) combination study in RA. The results are significant because they are the first data from a large Phase III trial to show that a humanized antibody targeted at B-cells improves the signs and symptoms of rheumatoid arthritis. Hope patients suffering from RA and those are not responding will breathe a sigh of relief in the days to come...
Many monoclonal antibodies like infliximab, etanercept and adalimumab were tried for the rheumatoid arthritis now its interseting to note that Genentech and Biogen Idec reported positive outcome from ocrelizumab ( humanized anti-CD20) -MTX (Methotrexate - see the structure : this drug is a part of DMARD treatment meant for RA patients) combination study in RA. The results are significant because they are the first data from a large Phase III trial to show that a humanized antibody targeted at B-cells improves the signs and symptoms of rheumatoid arthritis. Hope patients suffering from RA and those are not responding will breathe a sigh of relief in the days to come...Ref : http://www.gene.com/gene/news/press-releases/display.do?method=detail&id=12487
Tuesday, July 26, 2016
Anti-inflammatory drugs may improve severity of depressive symptoms, study finds
Monday, November 24, 2014
New drug combination shows promise as effective, safe treatment for rheumatoid arthritis
Monday, May 9, 2011
Pfizer RA Drug Meets Study Goals
Thursday, August 23, 2018
FDA Advisory Committee Recommends the Approval of Baricitinib 2mg, but not 4mg, for the Treatment of Moderately-to-Severely Active Rheumatoid Arthritis
"We are confident that baricitinib, if approved, can help people in the U.S. manage the challenges of living with RA," said Christi Shaw, president of Lilly Bio-Medicines. "While we are disappointed with the Advisory Committee's assessment of the data for the 4-mg dose, we are confident in the positive benefit-risk profile of both the 2-mg and the 4-mg doses. We look forward to continuing our work with the FDA on our New Drug Application (NDA) and are hopeful that baricitinib will receive approval in the coming months."
"Despite advances in the management of RA over the last 20 years, which include early treatment, optimized use of traditional therapies for rheumatic disease and the advent of newer medications such as biologics, many patients are still struggling to meet treatment targets, and live with debilitating pain, fatigue and other symptoms of RA," said Peter Taylor, MA, PhD, professor, University of Oxford, an expert who attended the Advisory Committee Meeting. "Baricitinib could be a promising option for RA patients in the U.S. who are not achieving adequate disease control with currently available treatments."
Wednesday, December 2, 2009
CCII capsules offer safe and effective treatment for rheumatoid arthritis
More....CCII capsules offer safe and effective treatment for rheumatoid arthritis
Wednesday, May 23, 2012
Pfizer seeks FDA support for its new anti-rheumatoid arthritis pill
Thursday, November 23, 2017
Methotrexate drug holiday improves flu vaccine efficacy in rheumatoid arthritis patients
In continuation of my update on methotrexate

People with RA who stop taking methotrexate treatment for just two weeks after they have a seasonal flu shot can improve the vaccine's efficacy without increasing RA disease activity, according to new research findings presented this week at the 2017 ACR/ARHP Annual Meeting in San Diego.
Thursday, August 26, 2010
FDA approves Chelsea Therapeutics' Phase II protocol for CH-4051 antifolate in rheumatoid arthritis
"Although MTX is considered the standard of care in RA, both as a monotherapy and in combination with other RA treatments, the dosing and maximal therapeutic benefit of MTX is limited by well-documented tolerability issues, long-term safety concerns and variable bioavailability," commented Dr. Simon Pedder, president and CEO of Chelsea Therapeutics. "Given that CH-4051 is metabolically stable and that all of our preclinical and clinical work suggests enhanced absorption, dramatically increased potency and improved tolerability over MTX, we believe CH-4051 will be safe and highly efficacious in a historically treatment-resistant patient population."




